Ultraviolet (UV) and hydrogen peroxide activate ceramide-ER stress-AMPK signaling axis to promote retinal pigment epithelium (RPE) cell apoptosis
- PMID: 23685869
- PMCID: PMC3676843
- DOI: 10.3390/ijms140510355
Ultraviolet (UV) and hydrogen peroxide activate ceramide-ER stress-AMPK signaling axis to promote retinal pigment epithelium (RPE) cell apoptosis
Abstract
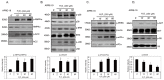
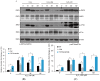
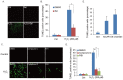
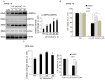
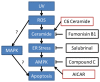
Ultraviolet (UV) radiation and reactive oxygen species (ROS) impair the physiological functions of retinal pigment epithelium (RPE) cells by inducing cell apoptosis, which is the main cause of age-related macular degeneration (AMD). The mechanism by which UV/ROS induces RPE cell death is not fully addressed. Here, we observed the activation of a ceramide-endoplasmic reticulum (ER) stress-AMP activated protein kinase (AMPK) signaling axis in UV and hydrogen peroxide (H2O2)-treated RPE cells. UV and H2O2 induced an early ceramide production, profound ER stress and AMPK activation. Pharmacological inhibitors against ER stress (salubrinal), ceramide production (fumonisin B1) and AMPK activation (compound C) suppressed UV- and H2O2-induced RPE cell apoptosis. Conversely, cell permeable short-chain C6 ceramide and AMPK activator AICAR (5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide) mimicked UV and H2O2's effects and promoted RPE cell apoptosis. Together, these results suggest that UV/H2O2 activates the ceramide-ER stress-AMPK signaling axis to promote RPE cell apoptosis.
Figures

References
-
- Friedman D.S., O’Colmain B.J., Munoz B., Tomany S.C., McCarty C., de Jong P.T., Nemesure B., Mitchell P., Kempen J. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. - PubMed
-
- Roduit R., Schorderet D.F. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis. 2008;13:343–353. - PubMed
-
- Liang Y.G., Jorgensen A.G., Kaestel C.G., Wiencke A.K., Lui G.M., la Cour M.H., Ropke C.H., Nissen M.H. Bcl-2, Bax, and c-Fos expression correlates to RPE cell apoptosis induced by UV-light and daunorubicin. Curr. Eye Res. 2000;20:25–34. - PubMed
-
- Nilsson S.E., Sundelin S.P., Wihlmark U., Brunk U.T. Aging of cultured retinal pigment epithelial cells: Oxidative reactions, lipofuscin formation and blue light damage. Doc. Ophthalmol. 2003;106:13–16. - PubMed
-
- Young R.W. Solar radiation and age-related macular degeneration. Surv. Ophthalmol. 1988;32:252–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

