Meroterpenes from marine invertebrates: structures, occurrence, and ecological implications
- PMID: 23685889
- PMCID: PMC3707164
- DOI: 10.3390/md11051602
Meroterpenes from marine invertebrates: structures, occurrence, and ecological implications
Abstract
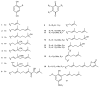
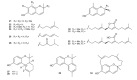
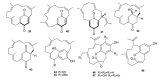
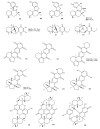
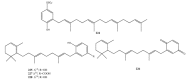
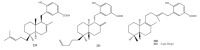
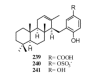
Meroterpenes are widely distributed among marine organisms; they are particularly abundant within brown algae, but other important sources include microorganisms and invertebrates. In the present review the structures and bioactivities of meroterpenes from marine invertebrates, mainly sponges and tunicates, are summarized. More than 300 molecules, often complex and with unique skeletons originating from intra- and inter-molecular cyclizations, and/or rearrangements, are illustrated. The reported syntheses are mentioned. The issue of a potential microbial link to their biosynthesis is also shortly outlined.
Figures

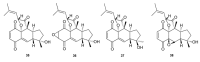






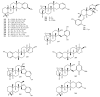


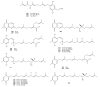
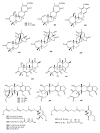
References
-
- Thomson R.H. Naturally Occurring Quinones. 2nd ed. Academic Press; London, UK: 1971. pp. 93–197.
-
- Pennock J.F. In: Terpenoids in Plants. Pridham J.B., editor. Academic Press; London, UK: 1967. pp. 129–146.
-
- Zubìa E., Ortega M., Salvà J. Natural products chemistry in marine ascidians of the genus Aplidium. Mini Rev. Org. Chem. 2005;2:546–564.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

