Stiffness, working stroke, and force of single-myosin molecules in skeletal muscle: elucidation of these mechanical properties via nonlinear elasticity evaluation
- PMID: 23685901
- PMCID: PMC11113998
- DOI: 10.1007/s00018-013-1353-x
Stiffness, working stroke, and force of single-myosin molecules in skeletal muscle: elucidation of these mechanical properties via nonlinear elasticity evaluation
Abstract
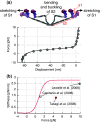
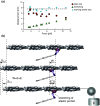
In muscles, the arrays of skeletal myosin molecules interact with actin filaments and continuously generate force at various contraction speeds. Therefore, it is crucial for myosin molecules to generate force collectively and minimize the interference between individual myosin molecules. Knowledge of the elasticity of myosin molecules is crucial for understanding the molecular mechanisms of muscle contractions because elasticity directly affects the working and drag (resistance) force generation when myosin molecules are positively or negatively strained. The working stroke distance is also an important mechanical property necessary for elucidation of the thermodynamic efficiency of muscle contractions at the molecular level. In this review, we focus on these mechanical properties obtained from single-fiber and single-molecule studies and discuss recent findings associated with these mechanical properties. We also discuss the potential molecular mechanisms associated with reduction of the drag effect caused by negatively strained myosin molecules.
Figures

Similar articles
-
Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments.Science. 2010 Aug 6;329(5992):686-9. doi: 10.1126/science.1191484. Science. 2010. PMID: 20689017
-
The size and the speed of the working stroke of muscle myosin and its dependence on the force.J Physiol. 2002 Nov 15;545(1):145-51. doi: 10.1113/jphysiol.2002.028969. J Physiol. 2002. PMID: 12433956 Free PMC article.
-
X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction.Biophys J. 1994 Dec;67(6):2422-35. doi: 10.1016/S0006-3495(94)80729-5. Biophys J. 1994. PMID: 7779179 Free PMC article.
-
X-ray diffraction studies of the contractile mechanism in single muscle fibres.Philos Trans R Soc Lond B Biol Sci. 2004 Dec 29;359(1452):1883-93. doi: 10.1098/rstb.2004.1557. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15647164 Free PMC article. Review.
-
Crossbridge and filament compliance in muscle: implications for tension generation and lever arm swing.J Muscle Res Cell Motil. 2010 Dec;31(4):245-65. doi: 10.1007/s10974-010-9232-7. Epub 2010 Dec 4. J Muscle Res Cell Motil. 2010. PMID: 21132353 Review.
Cited by
-
Do Actomyosin Single-Molecule Mechanics Data Predict Mechanics of Contracting Muscle?Int J Mol Sci. 2018 Jun 25;19(7):1863. doi: 10.3390/ijms19071863. Int J Mol Sci. 2018. PMID: 29941816 Free PMC article. Review.
-
Ensemble velocity of non-processive molecular motors with multiple chemical states.Interface Focus. 2014 Dec 6;4(6):20140032. doi: 10.1098/rsfs.2014.0032. Interface Focus. 2014. PMID: 25485083 Free PMC article.
-
Comparative analysis of widely used methods to remove nonfunctional myosin heads for the in vitro motility assay.J Muscle Res Cell Motil. 2018 Dec;39(5-6):175-187. doi: 10.1007/s10974-019-09505-1. Epub 2019 Mar 8. J Muscle Res Cell Motil. 2018. PMID: 30850933 Free PMC article.
-
Titin-mediated thick filament activation, through a mechanosensing mechanism, introduces sarcomere-length dependencies in mathematical models of rat trabecula and whole ventricle.Sci Rep. 2017 Jul 17;7(1):5546. doi: 10.1038/s41598-017-05999-2. Sci Rep. 2017. PMID: 28717163 Free PMC article.
-
Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices.Nat Cell Biol. 2016 Jan;18(1):33-42. doi: 10.1038/ncb3277. Epub 2015 Nov 30. Nat Cell Biol. 2016. PMID: 26619148 Free PMC article.
References
-
- Huxley AF, Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;173(4412):971–973. - PubMed
-
- Huxley H, Hanson J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954;173(4412):973–976. - PubMed
-
- Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

