Limited effects of exogenous glucose during severe hypoxia and a lack of hypoxia-stimulated glucose uptake in isolated rainbow trout cardiac muscle
- PMID: 23685969
- PMCID: PMC3749905
- DOI: 10.1242/jeb.085688
Limited effects of exogenous glucose during severe hypoxia and a lack of hypoxia-stimulated glucose uptake in isolated rainbow trout cardiac muscle
Abstract
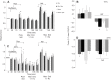
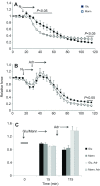
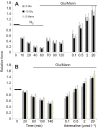
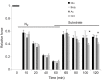
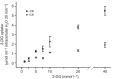
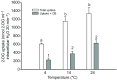
We examined whether exogenous glucose affects contractile performance of electrically paced ventricle strips from rainbow trout under conditions known to alter cardiomyocyte performance, ion regulation and energy demands. Physiological levels of d-glucose did not influence twitch force development for aerobic preparations (1) paced at 0.5 or 1.1 Hz, (2) at 15 or 23°C, (3) receiving adrenergic stimulation or (4) during reoxygenation with or without adrenaline after severe hypoxia. Contractile responses to ryanodine, an inhibitor of Ca(2+) release from the sarcoplasmic reticulum, were also not affected by exogenous glucose. However, glucose did attenuate the fall in twitch force during severe hypoxia. Glucose uptake was assayed in non-contracting ventricle strips using 2-[(3)H] deoxy-d-glucose (2-DG) under aerobic and hypoxic conditions, at different incubation temperatures and with different inhibitors. Based upon a lack of saturation of 2-DG uptake and incomplete inhibition of uptake by cytochalasin B and d-glucose, 2-DG uptake was mediated by a combination of facilitated transport and simple diffusion. Hypoxia stimulated lactate efflux sixfold to sevenfold with glucose present, but did not increase 2-DG uptake or reduce lactate efflux in the presence of cytochalasin B. Increasing temperature (14 to 24°C) also did not increase 2-DG uptake, but decreasing temperature (14 to 4°C) reduced 2-DG uptake by 45%. In conclusion, exogenous glucose improves mechanical performance under hypoxia but not under any of the aerobic conditions applied. The extracellular concentration of glucose and cold temperature appear to determine and limit cardiomyocyte glucose uptake, respectively, and together may help define a metabolic strategy that relies predominantly on intracellular energy stores.
Keywords: 2-deoxyglucose; adrenaline; contractility; glucose; heart; hypoxia; lactate; rainbow trout; reoxygenation.
Figures
References
-
- Anousis N., Carvalho R. A., Zhao P., Malloy C. R., Sherry A. D. (2004). Compartmentation of glycolysis and glycogenolysis in the perfused rat heart. NMR Biomed. 17, 51-59 - PubMed
-
- Arthur P. G., Keen J. E., Hochachka P. W., Farrell A. P. (1992). Metabolic state of the in situ perfused trout heart during severe hypoxia. Am. J. Physiol. 263, R798-R804 - PubMed
-
- Bailey J. R., Driedzic W. R. (1993). Influence of low-temperature acclimation on fate of metabolic fuels in rainbow trout (Oncorhynchus mykiss) hearts. Can. J. Zool. 71, 2167-2173
-
- Bailey J. R., Rodnick K. J., MacDougall R., Clowe S., Driedzic W. R. (2000a). Anoxic performance of the American eel (Anguilla rostrata L.) heart requires extracellular glucose. J. Exp. Zool. 286, 699-706 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

