Human papillomavirus infections: warts or cancer?
- PMID: 23685995
- PMCID: PMC3685896
- DOI: 10.1101/cshperspect.a012997
Human papillomavirus infections: warts or cancer?
Abstract
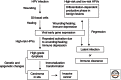
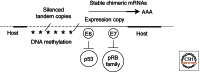
Human papillomaviruses (HPVs) are prevalent pathogens of mucosal and cutaneous epithelia. Productive infections of squamous epithelia lead to benign hyperproliferative warts, condylomata, or papillomas. Persistent infections of the anogenital mucosa by high-risk HPV genotypes 16 and 18 and closely related types can infrequently progress to high-grade intraepithelial neoplasias, carcinomas-in-situ, and invasive cancers in women and men. HPV-16 is also associated with a fraction of head and neck cancers. We discuss the interactions of the mucosotropic HPVs with the host regulatory proteins and pathways that lead to benign coexistence and enable HPV DNA amplification or, alternatively, to cancers that no longer support viral production.
Figures


References
-
- Belleudi F, Leone L, Purpura V, Cannella F, Scrofani C, Torrisi MR 2011. HPV16 E5 affects the KGFR/FGFR2b-mediated epithelial growth through alteration of the receptor expression, signaling and endocytic traffic. Oncogene 30: 4963–4876 - PubMed
-
- Bernard H-U 2002. Gene expression of genital human papillomaviruses and considerations on potential antiviral approaches. Antiviral Ther 7: 219–237 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
