Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling
- PMID: 23686370
- PMCID: PMC3698774
- DOI: 10.1161/JAHA.113.000140
Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling
Abstract
Background: Intramyocardial injection of mesenchymal stem cells (MSCs) in chronic ischemic cardiomyopathy is associated with reverse remodeling in experimental models and humans. Here, we tested the hypothesis that allogeneic MSC therapy drives ventricular remodeling by producing durable and progressive scar size reduction in ischemic cardiomyopathy.
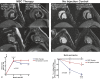
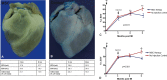
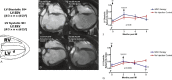
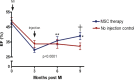
Methods and results: Gottingen swine (n=12) underwent left anterior descending coronary artery myocardial infarction (MI), and 3 months post-MI animals received either intramyocardial allogeneic MSC injection (200 mol/L cells; n=6) or left ventricle (LV) catheterization without injection (n=6). Swine were followed with serial cardiac magnetic resonance imaging for 9 months to assess structural and functional changes of the LV. Intramyocardial injection was performed using an integrated imaging platform combining electroanatomical mapping unipolar voltage and 3-dimensional cardiac magnetic resonance imaging angiography-derived anatomy to accurately target infarct border zone injections. MSC-treated animals had a 19.62 ± 2.86% reduction in scar size at 3 months postinjection, which progressed to 28.09 ± 2.31% from 3 to 6 months postinjection (P<0.0001). MSC-treated animals had unchanged end-diastolic volume (EDV; P=0.08) and end-systolic volume (ESV; P=0.28) from preinjection to 6 months postinjection, whereas controls had progressive dilatation in both EDV (P=0.0002) and ESV (P=0.0002). In addition, MSC-treated animals had improved LV sphericity index. Percentage change in infarct size correlated with percentage change in EDV (r=0.68; P=0.01) and ESV (r=0.77; P=0.001). Ejection fraction increased from 29.69 ± 1.68% to 35.85 ± 2.74% at 3 months post-MSC injection and progressed to 39.02 ± 2.42% 6 months postinjection (P=0.0001), whereas controls had a persistently depressed ejection fraction during follow-up (P=0.33).
Conclusion: Intramyocardial injection of allogeneic MSCs leads to a sustained and progressive reduction in infarct size, which in turn drives reverse remodeling and increases in ejection fraction. These findings support ongoing biological activity of cell therapy for substantial periods and suggest optimal end points for future clinical trials.
Keywords: cardiac magnetic resonance imaging; ischemic cardiomyopathy; stem cell therapy.
Figures


References
-
- Braunwald E, Pfeffer MA. Ventricular enlargement and remodeling following acute myocardial infarction: mechanisms and management. Am J Cardiol. 1991; 68:1D-6D - PubMed
-
- Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, de SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010; 121:948-954 - PubMed
-
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990; 81:1161-1172 - PubMed
-
- Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011; 4:98-108 - PubMed
-
- Lee TH, Hamilton MA, Stevenson LW, Moriguchi JD, Fonarow GC, Child JS, Laks H, Walden JA. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol. 1993; 72:672-676 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

