Aliskiren effect on plaque progression in established atherosclerosis using high resolution 3D MRI (ALPINE): a double-blind placebo-controlled trial
- PMID: 23686372
- PMCID: PMC3698800
- DOI: 10.1161/JAHA.112.004879
Aliskiren effect on plaque progression in established atherosclerosis using high resolution 3D MRI (ALPINE): a double-blind placebo-controlled trial
Abstract
Background: The renin-angiotensin system is well recognized as a mediator of pathophysiological events in atherosclerosis. The benefits of renin inhibition in atherosclerosis, especially when used in combination with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) are currently not known. We hypothesized that treatment with the renin inhibitor aliskiren in patients with established cardiovascular disease will prevent the progression of atherosclerosis as determined by high-resolution magnetic resonance imaging (MRI) measurements of arterial wall volume in the thoracic and abdominal aortas of high-risk patients with preexisting cardiovascular disease.
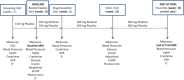
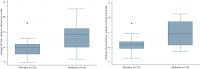
Methods and results: This was a single-center, randomized, double-blind, placebo-controlled trial in patients with established cardiovascular disease. After a 2-week single-blind placebo phase, patients were randomized to receive either placebo (n=37, mean ± SD age 64.5 ± 8.9 years, 3 women) or 150 mg of aliskiren (n=34, mean ± SD age 63.9 ± 11.5 years, 9 women). Treatment dose was escalated to 300 mg at 2 weeks and maintained during the remainder of the study. Patients underwent dark-blood, 3-dimensional MRI assessment of atherosclerotic plaque in the thoracic and abdominal segments at baseline and on study completion or termination (up to 36 weeks of drug or matching placebo). Aliskiren use resulted in significant progression of aortic wall volume (normalized total wall volume 5.31 ± 6.57 vs 0.15 ± 4.39 mm(3), P=0.03, and percentage wall volume 3.37 ± 2.96% vs 0.97 ± 2.02%, P=0.04) compared with placebo. In a subgroup analysis of subjects receiving ACEI/ARB therapy, atherosclerosis progression was observed only in the aliskiren group, not in the placebo group.
Conclusions: MRI quantification of atheroma plaque burden demonstrated that aliskiren use in patients with preexisting cardiovascular disease resulted in an unexpected increase in aortic atherosclerosis compared with placebo. Although preliminary, these results may have implications for the use of renin inhibition as a therapeutic strategy in patients with cardiovascular disease, especially in those receiving ACEI/ARB therapy.
Clinical trial registration: URL: http://ClinicalTrials.gov Unique identifier: NCT01417104.
Keywords: atherosclerosis; magnetic resonance imaging; renin.
Figures



References
-
- Dzau VJ. Theodore Cooper Lecture: tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047-1052 - PubMed
-
- Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system – focusing on the vascular system. Peptides. 2011;32:2141-2150 - PubMed
-
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators [see comments] [published erratum appears in N Engl J Med 2000;342:748]. N Engl J Med. 2000;342:145-153 - PubMed
-
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995-1003 - PubMed
-
- Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going?. J Hypertens. 2006;24:243-256 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

