Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice
- PMID: 23686747
- PMCID: PMC3715560
- DOI: 10.1002/jnr.23242
Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice
Abstract
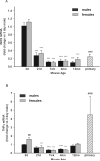
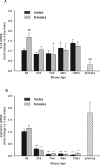
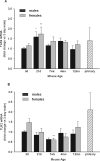
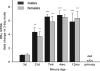
Although microglial activation is associated with all CNS disorders, many of which are sexually dimorphic or age-dependent, little is known about whether microglial basal gene expression is altered with age in the healthy CNS or whether it is sex dependent. Analysis of microglia from the brains of 3-day (P3)- to 12-month-old male and female C57Bl/6 mice revealed distinct gene expression profiles during postnatal development that differ significantly from those in adulthood. Microglia at P3 are characterized by relatively high iNOS, TNFα and arginase-I mRNA levels, whereas P21 microglia have increased expression of CD11b, TLR4, and FcRγI. Adult microglia (2-4 months) are characterized by low proinflammatory cytokine expression, which increases by 12 months of age. Age-dependent differences in gene expression suggest that microglia likely undergo phenotypic changes during ontogenesis, although in the healthy brain they did not express exclusively either M1 or M2 phenotypic markers at any time. Interestingly, microglia were sexually dimorphic only at P3, when females had higher expression of inflammatory cytokines than males, although there were no sex differences in estrogen receptor expression at this or any other time evaluated here. Compared with microglia in vivo, primary microglia prepared from P3 mice had considerably altered gene expression, with higher levels of TNFα, CD11b, arginase-I, and VEGF, suggesting that culturing may significantly alter microglial properties. In conclusion, age- and sex-specific variances in basal gene expression may allow differential microglial responses to the same stimulus at different ages, perhaps contributing to altered CNS vulnerabilities and/or disease courses.
Keywords: M1/M2 phenotype; aging; development; microglia; sexual dimorphism.
Copyright © 2013 Wiley Periodicals, Inc.
Figures



References
-
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. Journal of neuroendocrinology. 2012;24(1):183–190. - PubMed
-
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–5032. - PubMed
-
- Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase). Trends Neurosci. 1998;21(6):243–249. - PubMed
-
- Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Current opinion in clinical nutrition and metabolic care. 2003;6(2):223–228. - PubMed
-
- Bloch GJ, Kurth SM, Akesson TR, Micevych PE. Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin-immunoreactive cells. Brain Res. 1992;595(2):301–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

