A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study
- PMID: 23687260
- PMCID: PMC3786641
- DOI: 10.1136/annrheumdis-2012-203090
A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study
Abstract
Objectives: To compare the efficacy and safety of innovator infliximab (INX) and CT-P13, an INX biosimilar, in active rheumatoid arthritis patients with inadequate response to methotrexate (MTX) treatment.
Methods: Phase III randomised, double-blind, multicentre, multinational, parallel-group study. Patients with active disease despite MTX (12.5-25 mg/week) were randomised to receive 3 mg/kg of CT-P13 (n=302) or INX (n=304) with MTX and folic acid. The primary endpoint was the American College of Rheumatology 20% (ACR20) response at week 30. Therapeutic equivalence of clinical response according to ACR20 criteria was concluded if the 95% CI for the treatment difference was within ±15%. Secondary endpoints included ACR response criteria, European League Against Rheumatism (EULAR) response criteria, change in Disease Activity Score 28 (DAS28), Medical Outcomes Study Short-Form Health Survey (SF-36), Simplified Disease Activity Index, Clinical Disease Activity Index, as well as pharmacokinetic (PK) and pharmacodynamic (PD) parameters, safety and immunogenicity.
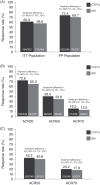
Results: At week 30, ACR20 responses were 60.9% for CT-P13 and 58.6% for INX (95% CI -6% to 10%) in the intention-to-treat population. The proportions in CT-P13 and INX groups achieving good or moderate EULAR responses (C reactive protein (CRP)) at week 30 were 85.8% and 87.1%, respectively. Low disease activity or remission according to DAS28-CRP, ACR-EULAR remission rates, ACR50/ACR70 responses and all other PK and PD endpoints were highly similar at week 30. Incidence of drug-related adverse events (35.2% vs 35.9%) and detection of antidrug antibodies (48.4% vs 48.2%) were highly similar for CT-P13 and INX, respectively.
Conclusions: CT-P13 demonstrated equivalent efficacy to INX at week 30, with a comparable PK profile and immunogenicity. CT-P13 was well tolerated, with a safety profile comparable with that of INX. CLINICALTRIALS.GOV IDENTIFIER: NCT01217086.
Figures

Comment in
-
Biosimilars to treat inflammatory arthritis: the challenge of proving identity.Ann Rheum Dis. 2013 Oct;72(10):1589-93. doi: 10.1136/annrheumdis-2012-203198. Epub 2013 Jul 29. Ann Rheum Dis. 2013. PMID: 23897773 No abstract available.
References
-
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9 - PubMed
-
- Dörner T, Strand V, Castañeda-Hernández G, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis 2013;72:322–8 - PubMed
-
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicenter, parallel-group, prospective phase 1 study comparing the pharmacokinetics, safety and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS study. Ann Rheum Dis 2013. doi:10.1136/annrheumdis-2012-203091 - PMC - PubMed
-
- Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13 - PubMed
-
- World Health Organization Commercial serodiagnostic tests for diagnosis of tuberculosis: policy statement. Geneva: WHO, 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

