The protective effect of antioxidants on orbital fibroblasts from patients with Graves' ophthalmopathy in response to oxidative stress
- PMID: 23687429
- PMCID: PMC3654843
The protective effect of antioxidants on orbital fibroblasts from patients with Graves' ophthalmopathy in response to oxidative stress
Abstract
Purpose: To investigate the biphasic effects of hydrogen peroxide (H2O2) on the orbital fibroblasts of patients with Graves' ophthalmopathy (GO) and the relation to antioxidants and proinflammatory cytokines.
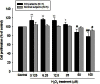
Methods: Proliferation of cultured orbital fibroblasts from patients with GO and normal controls was evaluated in response to various concentrations of H2O2. The effect of low concentrations of H2O2 (6.25 μM) on the cellular proliferation and induction of intracellular proinflammatory cytokines, and reactive oxygen species of orbital fibroblasts were assessed. Protective effects of N-acetylcysteine and vitamin C on GO fibroblasts in response to 6.25 μM H2O2 stimulation were also investigated.
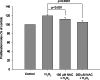
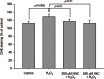
Results: When the GO fibroblasts were exposed to H2O2 at a concentration of 50 μM or above, significant cytotoxicity was observed. In contrast, lower concentrations of H2O2 (3.125-25 μM) increased the survival of GO fibroblasts with the peak cellular proliferation at 6.25 μM H2O2. However, this biphasic effect of H2O2 on the viability of orbital fibroblasts was not found in normal controls. In addition, 6.25 μM H2O2 led to significant elevation of the levels of transforming growth factor, beta 1, interleukin-1β, and superoxide anion in GO fibroblasts, but no significant change in the normal controls. Pretreatment with N-acetylcysteine or vitamin C reversed the enhanced proliferation capacity and the induction of transforming growth factor, beta 1, interleukin-1β and superoxide anion of GO fibroblasts in response to 6.25 μM H2O2.
Conclusions: These findings revealed the biphasic effect of H2O2 on cellular proliferation of GO orbital fibroblasts. Importantly, a low level of H2O2 can stimulate proliferation of GO orbital fibroblasts and induce the production of proinflammatory cytokines, which can be inhibited by pretreatment with antioxidants. This provides a theoretical basis for the rational use of antioxidant in treating GO at an early stage.
Figures


References
-
- Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120:380–6. - PubMed
-
- Smith TJ, Tsai CC, Shih MJ, Tsui S, Chen B, Han R, Naik V, King CS, Press C, Kamat S, Goldberg RA, Phipps RP, Douglas RS, Gianoukakis AG. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008;18:983–8. - PMC - PubMed
-
- Bartalena L, Tanda ML, Piantanida E, Lai A. Oxidative stress and Graves' ophthalmopathy: in vitro studies and therapeutic implications. Biofactors. 2003;19:155–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
