Drug-like property profiling of novel neuroprotective compounds to treat acute ischemic stroke: guidelines to develop pleiotropic molecules
- PMID: 23687519
- PMCID: PMC3653324
- DOI: 10.1007/s12975-012-0200-y
Drug-like property profiling of novel neuroprotective compounds to treat acute ischemic stroke: guidelines to develop pleiotropic molecules
Abstract
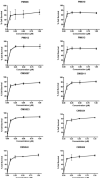
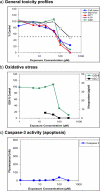
The development of novel neuroprotective compounds to treat acute ischemic stroke (AIS) has been problematic and quite complicated, since many candidates that have been tested clinically lacked significant pleiotropic activity, were unable to effectively cross the blood brain barrier (BBB), had poor bioavailability or were toxic. Moreover, the compounds did not confer significant neuroprotection or clinical efficacy measured using standard behavioral endpoints, when studied in clinical trials in a heterogeneous population of stroke patients. To circumvent some of the drug development problems describe above, we have used a rational funnel approach to identify and develop promising candidates. Using a step-wise approach, we have identified a series of compounds based upon two different neuroprotection assays. We have then taken the candidates and determined their "drug-like" properties. This guidelines article details in vitro screening assays used to show pleiotropic activity of a series of novel compounds; including enhanced neuroprotective activity compared to the parent compound fisetin. Moreover, for preliminary drug de-risking or risk reduction during development, we used compound assessment in the CeeTox assay, ADME toxicity using the AMES test for genotoxicity and interaction with Cytochrome P450 using CYP450 inhibition analysis against a spectrum of CYP450 enzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4) as a measure of drug interaction. Moreover, the compounds have been studied using a transfected Madin Darby canine kidney (MDCK) cell assay to assess blood brain barrier penetration (BBB). Using this series of assays, we have identified 4 novel molecules to be developed as an AIS treatment.
Keywords: ADME; Ames test; MDCK; chalcone; flavone; flavonoid; pleiotropic; screening; toxicity.
Figures
Similar articles
-
A series of novel neuroprotective blood brain barrier penetrating flavonoid drugs to treat acute ischemic stroke.Curr Pharm Des. 2012;18(25):3694-703. doi: 10.2174/138161212802002652. Curr Pharm Des. 2012. PMID: 22574983 Review.
-
A novel approach to screening for new neuroprotective compounds for the treatment of stroke.Brain Res. 2007 Oct 10;1173:117-25. doi: 10.1016/j.brainres.2007.07.061. Epub 2007 Aug 9. Brain Res. 2007. PMID: 17765210 Free PMC article.
-
Cytochrome P450-mediated herb-drug interaction (HDI) of Polygonum multiflorum Thunb. based on pharmacokinetic studies and in vitro inhibition assays.Phytomedicine. 2023 Apr;112:154710. doi: 10.1016/j.phymed.2023.154710. Epub 2023 Feb 9. Phytomedicine. 2023. PMID: 36805481
-
Development of Robust Quantitative Structure-Activity Relationship Models for CYP2C9, CYP2D6, and CYP3A4 Catalysis and Inhibition.Drug Metab Dispos. 2021 Sep;49(9):822-832. doi: 10.1124/dmd.120.000320. Epub 2021 Jun 28. Drug Metab Dispos. 2021. PMID: 34183376 Free PMC article.
-
Clinical trials in acute ischemic stroke.CNS Drugs. 2014 Oct;28(10):929-38. doi: 10.1007/s40263-014-0199-6. CNS Drugs. 2014. PMID: 25160686 Review.
Cited by
-
The Neuroprotective Role of Fisetin in Different Neurological Diseases: a Systematic Review.Mol Neurobiol. 2023 Nov;60(11):6383-6394. doi: 10.1007/s12035-023-03469-7. Epub 2023 Jul 15. Mol Neurobiol. 2023. PMID: 37453993
-
Cytoprotective Drug-Tissue Plasminogen Activator Protease Interaction Assays: Screening of Two Novel Cytoprotective Chromones.Transl Stroke Res. 2017 Apr 12. doi: 10.1007/s12975-017-0533-7. Online ahead of print. Transl Stroke Res. 2017. PMID: 28405804
-
A cost-effective rabbit embolic stroke bioassay: insight into the development of acute ischemic stroke therapy.Transl Stroke Res. 2015 Apr;6(2):99-103. doi: 10.1007/s12975-015-0386-x. Epub 2015 Feb 1. Transl Stroke Res. 2015. PMID: 25637174 Free PMC article. No abstract available.
-
Absence of genotoxic effects of the chalcone (E)-1-(2-hydroxyphenyl)-3-(4-methylphenyl)-prop-2-en-1-one) and its potential chemoprevention against DNA damage using in vitro and in vivo assays.PLoS One. 2017 Feb 16;12(2):e0171224. doi: 10.1371/journal.pone.0171224. eCollection 2017. PLoS One. 2017. PMID: 28207781 Free PMC article.
-
J-147 a Novel Hydrazide Lead Compound to Treat Neurodegeneration: CeeTox™ Safety and Genotoxicity Analysis.J Neurol Neurophysiol. 2013 Aug;4(3):158. doi: 10.4172/2155-9562.1000158. J Neurol Neurophysiol. 2013. PMID: 25364619 Free PMC article.
References
-
- Bernas T, Dobrucki J. Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry. 2002 Apr 1;47(4):236–42. - PubMed
-
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993 Jun;303(2):474–82. - PubMed
-
- Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007 Dec 12;150(3):585–91. - PubMed
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics—2012 Update. Circulation. 2011 Dec 15;2011 DOI:10.1161/CIR.0b013e31823ac046.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

