Computational models and emergent properties of respiratory neural networks
- PMID: 23687564
- PMCID: PMC3656479
- DOI: 10.1002/cphy.c110016
Computational models and emergent properties of respiratory neural networks
Abstract
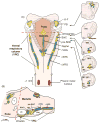
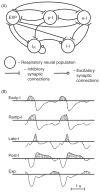
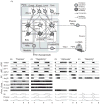
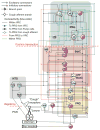
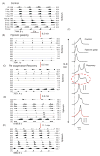
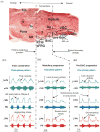
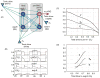
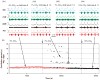
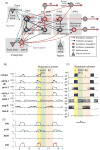
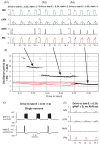
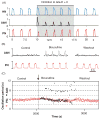
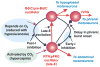
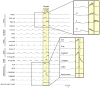
Computational models of the neural control system for breathing in mammals provide a theoretical and computational framework bringing together experimental data obtained from different animal preparations under various experimental conditions. Many of these models were developed in parallel and iteratively with experimental studies and provided predictions guiding new experiments. This data-driven modeling approach has advanced our understanding of respiratory network architecture and neural mechanisms underlying generation of the respiratory rhythm and pattern, including their functional reorganization under different physiological conditions. Models reviewed here vary in neurobiological details and computational complexity and span multiple spatiotemporal scales of respiratory control mechanisms. Recent models describe interacting populations of respiratory neurons spatially distributed within the Bötzinger and pre-Bötzinger complexes and rostral ventrolateral medulla that contain core circuits of the respiratory central pattern generator (CPG). Network interactions within these circuits along with intrinsic rhythmogenic properties of neurons form a hierarchy of multiple rhythm generation mechanisms. The functional expression of these mechanisms is controlled by input drives from other brainstem components,including the retrotrapezoid nucleus and pons, which regulate the dynamic behavior of the core circuitry. The emerging view is that the brainstem respiratory network has rhythmogenic capabilities at multiple levels of circuit organization. This allows flexible, state-dependent expression of different neural pattern-generation mechanisms under various physiological conditions,enabling a wide repertoire of respiratory behaviors. Some models consider control of the respiratory CPG by pulmonary feedback and network reconfiguration during defensive behaviors such as cough. Future directions in modeling of the respiratory CPG are considered.
Figures










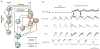

Similar articles
-
Structural and functional architecture of respiratory networks in the mammalian brainstem.Philos Trans R Soc Lond B Biol Sci. 2009 Sep 12;364(1529):2577-87. doi: 10.1098/rstb.2009.0081. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19651658 Free PMC article. Review.
-
Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms.J Neurophysiol. 2007 Dec;98(6):3370-87. doi: 10.1152/jn.00985.2007. Epub 2007 Oct 3. J Neurophysiol. 2007. PMID: 17913982 Free PMC article.
-
Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation.Prog Brain Res. 2007;165:201-20. doi: 10.1016/S0079-6123(06)65013-9. Prog Brain Res. 2007. PMID: 17925248 Free PMC article. Review.
-
Respiratory rhythm and pattern generation: Brainstem cellular and circuit mechanisms.Handb Clin Neurol. 2022;188:1-35. doi: 10.1016/B978-0-323-91534-2.00004-7. Handb Clin Neurol. 2022. PMID: 35965022 Review.
-
Functional Interactions between Mammalian Respiratory Rhythmogenic and Premotor Circuitry.J Neurosci. 2016 Jul 6;36(27):7223-33. doi: 10.1523/JNEUROSCI.0296-16.2016. J Neurosci. 2016. PMID: 27383596 Free PMC article.
Cited by
-
Correlation Analysis of Synchronization Type and Degree in Respiratory Neural Network.Comput Intell Neurosci. 2021 Dec 27;2021:4475184. doi: 10.1155/2021/4475184. eCollection 2021. Comput Intell Neurosci. 2021. PMID: 34987564 Free PMC article.
-
Defining inhibitory neurone function in respiratory circuits: opportunities with optogenetics?J Physiol. 2015 Jul 15;593(14):3033-46. doi: 10.1113/jphysiol.2014.280610. Epub 2014 Dec 22. J Physiol. 2015. PMID: 25384785 Free PMC article. Review.
-
Apnea in acute bilirubin encephalopathy.Semin Perinatol. 2014 Nov;38(7):407-11. doi: 10.1053/j.semperi.2014.08.003. Epub 2014 Sep 17. Semin Perinatol. 2014. PMID: 25239473 Free PMC article. Review.
-
Multiscale fingerprinting of neuronal functional connectivity.Brain Struct Funct. 2015 Sep;220(5):2967-82. doi: 10.1007/s00429-014-0838-1. Epub 2014 Jul 24. Brain Struct Funct. 2015. PMID: 25056933 Free PMC article.
-
Respiratory neuron characterization reveals intrinsic bursting properties in isolated adult turtle brainstems (Trachemys scripta).Respir Physiol Neurobiol. 2016 Apr;224:52-61. doi: 10.1016/j.resp.2014.11.004. Epub 2014 Nov 13. Respir Physiol Neurobiol. 2016. PMID: 25462012 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

