The effect of the N-mesityl group in NHC-catalyzed reactions
- PMID: 23687565
- PMCID: PMC3655724
- DOI: 10.1039/C1SC00397F
The effect of the N-mesityl group in NHC-catalyzed reactions
Abstract
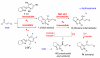
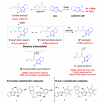
The majority of N-heterocyclic carbene catalyzed reactions of α-functionalized aldehydes, including annulations, oxidations, and redox reactions, occur more rapidly with N-mesityl substituted NHCs. In many cases, no reaction occurs with NHCs lacking ortho-substituted aromatics. By careful competition studies, catalyst analogue synthesis, mechanistic investigations, and consideration of the elementary steps in NHC-catalyzed reactions of enals, we have determined that the effect of the N-mesityl group is to render the initial addition of the NHC to the aldehyde irreversible, thereby accelerating the formation of the Breslow intermediate. These studies rationalize the experimentally observed catalyst preference for all classes of NHC-catalyzed reactions of aldehydes and provide a roadmap for catalyst selection and design.
Figures

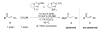





Similar articles
-
On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums.Acc Chem Res. 2014 Feb 18;47(2):696-707. doi: 10.1021/ar400239v. Epub 2014 Jan 10. Acc Chem Res. 2014. PMID: 24410291
-
NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles.Acc Chem Res. 2019 Feb 19;52(2):425-436. doi: 10.1021/acs.accounts.8b00550. Epub 2019 Jan 17. Acc Chem Res. 2019. PMID: 30653296
-
N-Heterocyclic carbene (NHC)-catalyzed oxidative [3+2] annulation of dioxindoles and enals: mechanism, role of NHC, role of a mixture of bases with different strength, and origin of stereoselectivity.Phys Chem Chem Phys. 2020 Dec 23;22(48):28269-28276. doi: 10.1039/d0cp05129b. Phys Chem Chem Phys. 2020. PMID: 33295368
-
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.Acc Chem Res. 2020 Mar 17;53(3):690-702. doi: 10.1021/acs.accounts.9b00635. Epub 2020 Mar 6. Acc Chem Res. 2020. PMID: 32142245 Review.
-
N-Heterocyclic Carbene (NHC)-Catalyzed Transformations Involving Azolium Enolates.Chem Rec. 2022 Aug;22(8):e202200054. doi: 10.1002/tcr.202200054. Epub 2022 May 13. Chem Rec. 2022. PMID: 35562645 Review.
Cited by
-
N-Heterocyclic carbene catalysed redox isomerisation of esters to functionalised benzaldehydes.Chem Sci. 2015 Apr 1;6(4):2366-2370. doi: 10.1039/c4sc03726j. Epub 2015 Jan 26. Chem Sci. 2015. PMID: 29308150 Free PMC article.
-
Tale of the Breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: then and now.Chem Sci. 2021 May 11;12(23):7973-7992. doi: 10.1039/d1sc01910d. Chem Sci. 2021. PMID: 34194690 Free PMC article. Review.
-
Carbene-Catalyzed Phthalide Ether Functionalization for Discovering Chiral Phytovirucide that Specifically Targets Viral Nia Protein to Inhibit Proliferation.Research (Wash D C). 2025 Mar 14;8:0637. doi: 10.34133/research.0637. eCollection 2025. Research (Wash D C). 2025. PMID: 40093974 Free PMC article.
-
SNAr-Derived Decomposition By-products Involving Pentafluorophenyl Triazolium Carbenes.Synlett. 2013 Jun 17;24(10):10.1055/s-0033-1338842. doi: 10.1055/s-0033-1338842. Synlett. 2013. PMID: 24379522 Free PMC article.
-
Rate and Equilibrium Constants for the Addition of N-Heterocyclic Carbenes into Benzaldehydes: A Remarkable 2-Substituent Effect.Angew Chem Weinheim Bergstr Ger. 2015 Jun 1;127(23):6991-6996. doi: 10.1002/ange.201501840. Epub 2015 Apr 23. Angew Chem Weinheim Bergstr Ger. 2015. PMID: 27478264 Free PMC article.
References
-
- Reynolds NT, Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2004;126:9518–9519. - PubMed
-
- Burstein C, Glorius F. Angew. Chem., Int. Ed. 2004;43:6205–6208. - PubMed
-
-
For recent reviews on NHC catalysis, see Chiang P-C, Bode JW. N-Heterocyclic Carbenes. The Royal Society of Chemistry; 2011. pp. 399–435. Moore JL, Rovis T. Top. Curr. Chem. 2009;291:77–144. Nair V, Vellalath S, Babu BP. Chem. Soc. Rev. 2008;37:2691–2698. Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655.
-
-
-
For the first report of a N-mesityl substituted triazolium precatalyst, see Sohn SS, Bode JW. Org. Lett. 2005;7:3873–3876. For the first chiral variant, see He M, Struble JR, Bode JW. J. Am. Chem. Soc. 2006;128:8418–8420. For the synthesis of N-mesityl triazolium salts, see: Struble JR, Bode JW. Org. Synth. 2010;87:362–376.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

