The effect of the N-mesityl group in NHC-catalyzed reactions
- PMID: 23687565
- PMCID: PMC3655724
- DOI: 10.1039/C1SC00397F
The effect of the N-mesityl group in NHC-catalyzed reactions
Abstract
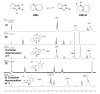
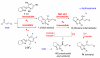
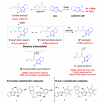
The majority of N-heterocyclic carbene catalyzed reactions of α-functionalized aldehydes, including annulations, oxidations, and redox reactions, occur more rapidly with N-mesityl substituted NHCs. In many cases, no reaction occurs with NHCs lacking ortho-substituted aromatics. By careful competition studies, catalyst analogue synthesis, mechanistic investigations, and consideration of the elementary steps in NHC-catalyzed reactions of enals, we have determined that the effect of the N-mesityl group is to render the initial addition of the NHC to the aldehyde irreversible, thereby accelerating the formation of the Breslow intermediate. These studies rationalize the experimentally observed catalyst preference for all classes of NHC-catalyzed reactions of aldehydes and provide a roadmap for catalyst selection and design.
Figures

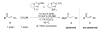





References
-
- Reynolds NT, Read de Alaniz J, Rovis T. J. Am. Chem. Soc. 2004;126:9518–9519. - PubMed
-
- Burstein C, Glorius F. Angew. Chem., Int. Ed. 2004;43:6205–6208. - PubMed
-
-
For recent reviews on NHC catalysis, see Chiang P-C, Bode JW. N-Heterocyclic Carbenes. The Royal Society of Chemistry; 2011. pp. 399–435. Moore JL, Rovis T. Top. Curr. Chem. 2009;291:77–144. Nair V, Vellalath S, Babu BP. Chem. Soc. Rev. 2008;37:2691–2698. Enders D, Niemeier O, Henseler A. Chem. Rev. 2007;107:5606–5655.
-
-
-
For the first report of a N-mesityl substituted triazolium precatalyst, see Sohn SS, Bode JW. Org. Lett. 2005;7:3873–3876. For the first chiral variant, see He M, Struble JR, Bode JW. J. Am. Chem. Soc. 2006;128:8418–8420. For the synthesis of N-mesityl triazolium salts, see: Struble JR, Bode JW. Org. Synth. 2010;87:362–376.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

