Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma
- PMID: 23687622
- PMCID: PMC3655740
- DOI: 10.4161/onci.23562
Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma
Abstract
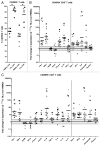
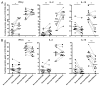
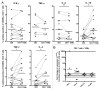
Although malignant cells can be recognized and controlled by the immune system, in patients with clinically apparent cancer immunosurveillance has failed. To better understand local immunoregulatory processes that impact on cancer progression, we correlated intratumoral immunological profiles with the survival of patients affected by primary clear cell renal cell carcinoma (ccRCC). A retrospective analysis of 54 primary ccRCC samples for 31 different immune response-related transcripts, revealed a negative correlation of CD68 (a marker of tumor-associated macrophages, TAMs) and FOXP3 (a marker of regulatory T cells, Tregs) with survival. The subsequent analysis of 12 TAM-related transcripts revealed an association between the genes coding for CD163, interferon regulatory factor 4 (IRF4) and fibronectin 1 (FN1), all of which have been linked to the M2 TAM phenotype, with reduced survival and increased tumor stage, whereas the opposite was the case for the M1-associated gene coding for inducible nitric oxide synthetase (iNOS). The M2 signature of (CD68+) TAMs was found to correlate with CD163 expression, as determined in prospectively collected fresh ccRCC tissue samples. Upon co-culture with autologous tumor cells, CD11b+ cells isolated from paired blood samples expressed CD163 and other M2-associated proteins, suggesting that the malignant cells promote the accumulation of M2 TAMs. Furthermore, the tumor-associated milieu as well as isolated TAMs induced the skewing of autologous, blood-derived CD4+ T cells toward a more immunosuppressive phenotype, as shown by decreased production of effector cytokines, increased production of interleukin-10 (IL-10) and enhanced expression of the co-inhibitory molecules programmed death 1 (PD-1) and T-cell immunoglobulin mucin 3 (TIM-3). Taken together, our data suggest that ccRCC progressively attracts macrophages and induces their skewing into M2 TAMs, in turn subverting tumor-infiltrating T cells such that immunoregulatory functions are increased at the expense of effector functions.
Keywords: T-cell response; clear cell renal cell carcinoma; immunoregulation; tumor immunology; tumor-associated macrophages.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
