Accumulation of inorganic polyphosphate enables stress endurance and catalytic vigour in Pseudomonas putida KT2440
- PMID: 23687963
- PMCID: PMC3673903
- DOI: 10.1186/1475-2859-12-50
Accumulation of inorganic polyphosphate enables stress endurance and catalytic vigour in Pseudomonas putida KT2440
Abstract
Background: Accumulation of inorganic polyphosphate (polyP), a persistent trait throughout the whole Tree of Life, is claimed to play a fundamental role in enduring environmental insults in a large variety of microorganisms. The share of polyP in the tolerance of the soil bacterium Pseudomonas putida KT2440 to a suite of physicochemical stresses has been studied on the background of its capacity as a host of oxidative biotransformations.
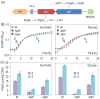
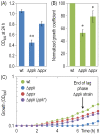
Results: Cells lacking polyphosphate kinase (Ppk), which expectedly presented a low intracellular polyP level, were more sensitive to a number of harsh external conditions such as ultraviolet irradiation, addition of β-lactam antibiotics and heavy metals (Cd(2+) and Cu(2+)). Other phenotypes related to a high-energy phosphate load (e.g., swimming) were substantially weakened as well. Furthermore, the ppk mutant was consistently less tolerant to solvents and its survival in stationary phase was significantly affected. In contrast, the major metabolic routes were not significantly influenced by the loss of Ppk as diagnosed from respiration patterns of the mutant in phenotypic microarrays. However, the catalytic vigour of the mutant decreased to about 50% of that in the wild-type strain as estimated from the specific growth rate of cells carrying the catabolic TOL plasmid pWW0 for m-xylene biodegradation. The catalytic phenotype of the mutant was restored by over-expressing ppk in trans. Some of these deficits could be explained by the effect of the ppk mutation on the expression profile of the rpoS gene, the stationary phase sigma factor, which was revealed by the analysis of a PrpoS → rpoS'-'lacZ translational fusion. Still, every stress-related effect of lacking Ppk in P. putida was relatively moderate as compared to some of the conspicuous phenotypes reported for other bacteria.
Conclusions: While polyP can be involved in a myriad of cellular functions, the polymer seems to play a relatively secondary role in the genetic and biochemical networks that ultimately enable P. putida to endure environmental stresses. Instead, the main value of polyP could be ensuring a reservoire of energy during prolonged starvation. This is perhaps one of the reasons for polyP persistence in live systems despite its apparent lack of essentiality.
Figures

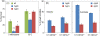


Similar articles
-
The RNA chaperone Hfq enables the environmental stress tolerance super-phenotype of Pseudomonas putida.Environ Microbiol. 2016 Oct;18(10):3309-3326. doi: 10.1111/1462-2920.13052. Epub 2015 Dec 2. Environ Microbiol. 2016. PMID: 26373442
-
The effect of polyphosphate kinase gene deletion on polyhydroxyalkanoate accumulation and carbon metabolism in Pseudomonas putida KT2440.Environ Microbiol Rep. 2013 Oct;5(5):740-6. doi: 10.1111/1758-2229.12076. Epub 2013 Aug 18. Environ Microbiol Rep. 2013. PMID: 24115625
-
Polyphosphate kinase of Lysinibacillus sphaericus and its effects on accumulation of polyphosphate and bacterial growth.Microbiol Res. 2015 Mar;172:41-7. doi: 10.1016/j.micres.2014.12.002. Epub 2014 Dec 12. Microbiol Res. 2015. PMID: 25541179
-
Inorganic polyphosphate: a molecule of many functions.Annu Rev Biochem. 1999;68:89-125. doi: 10.1146/annurev.biochem.68.1.89. Annu Rev Biochem. 1999. PMID: 10872445 Review.
-
Inorganic polyphosphate regulates responses of Escherichia coli to nutritional stringencies, environmental stresses and survival in the stationary phase.Prog Mol Subcell Biol. 1999;23:183-95. doi: 10.1007/978-3-642-58444-2_9. Prog Mol Subcell Biol. 1999. PMID: 10448677 Review.
Cited by
-
Leveraging Pseudomonas Stress Response Mechanisms for Industrial Applications.Front Microbiol. 2021 May 10;12:660134. doi: 10.3389/fmicb.2021.660134. eCollection 2021. Front Microbiol. 2021. PMID: 34040596 Free PMC article. Review.
-
A Stringent Analysis of Polyphosphate Dynamics in Escherichia coli.J Bacteriol. 2019 Apr 9;201(9):e00070-19. doi: 10.1128/JB.00070-19. Print 2019 May 1. J Bacteriol. 2019. PMID: 30782636 Free PMC article.
-
Pseudomonas putida KT2440 Strain Metabolizes Glucose through a Cycle Formed by Enzymes of the Entner-Doudoroff, Embden-Meyerhof-Parnas, and Pentose Phosphate Pathways.J Biol Chem. 2015 Oct 23;290(43):25920-32. doi: 10.1074/jbc.M115.687749. Epub 2015 Sep 8. J Biol Chem. 2015. PMID: 26350459 Free PMC article.
-
Proteins with CHADs (Conserved Histidine α-Helical Domains) Are Attached to Polyphosphate Granules In Vivo and Constitute a Novel Family of Polyphosphate-Associated Proteins (Phosins).Appl Environ Microbiol. 2017 Mar 17;83(7):e03399-16. doi: 10.1128/AEM.03399-16. Print 2017 Apr 1. Appl Environ Microbiol. 2017. PMID: 28130300 Free PMC article.
-
Is there a link between inorganic polyphosphate (polyP), mitochondria, and neurodegeneration?Pharmacol Res. 2021 Jan;163:105211. doi: 10.1016/j.phrs.2020.105211. Epub 2020 Oct 1. Pharmacol Res. 2021. PMID: 33010423 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

