Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis
- PMID: 23687968
- PMCID: PMC3663788
- DOI: 10.1186/1297-9716-44-37
Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis
Abstract
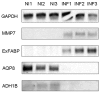
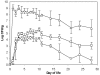
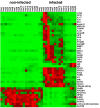
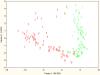
The characterization of the immune response of chickens to Salmonella infection is usually limited to the quantification of expression of genes coding for cytokines, chemokines or antimicrobial peptides. However, processes occurring in the cecum of infected chickens are likely to be much more diverse. In this study we have therefore characterized the transcriptome and proteome in the chicken cecum after infection with Salmonella Enteritidis. Using a combination of 454 pyrosequencing, protein mass spectrometry and quantitative real-time PCR, we identified 48 down- and 56 up-regulated chicken genes after Salmonella Enteritidis infection. The most inducible gene was that coding for MMP7, exhibiting a 5952 fold induction 9 days post-infection. An induction of greater than 100 fold was observed for IgG, IRG1, SAA, ExFABP, IL-22, TRAP6, MRP126, IFNγ, iNOS, ES1, IL-1β, LYG2, IFIT5, IL-17, AVD, AH221 and SERPIN B. Since prostaglandin D2 synthase was upregulated and degrading hydroxyprostaglandin dehydrogenase was downregulated after the infection, prostaglandin must accumulate in the cecum of chickens infected with Salmonella Enteritidis. Finally, above mentioned signaling was dependent on the presence of a SPI1-encoded type III secretion system in Salmonella Enteritidis. The inflammation lasted for 2 weeks after which time the expression of the "inflammatory" genes returned back to basal levels and, instead, the expression of IgA and IgG increased. This points to an important role for immunoglobulins in the restoration of homeostasis in the cecum after infection.
Figures
Similar articles
-
Characterization of chicken spleen transcriptome after infection with Salmonella enterica serovar Enteritidis.PLoS One. 2012;7(10):e48101. doi: 10.1371/journal.pone.0048101. Epub 2012 Oct 19. PLoS One. 2012. PMID: 23094107 Free PMC article.
-
Gene expression in the chicken caecum in response to infections with non-typhoid Salmonella.Vet Res. 2014 Dec 5;45(1):119. doi: 10.1186/s13567-014-0119-2. Vet Res. 2014. PMID: 25475706 Free PMC article. Review.
-
Chicken-Specific Kinome Array Reveals that Salmonella enterica Serovar Enteritidis Modulates Host Immune Signaling Pathways in the Cecum to Establish a Persistence Infection.Int J Mol Sci. 2016 Jul 27;17(8):1207. doi: 10.3390/ijms17081207. Int J Mol Sci. 2016. PMID: 27472318 Free PMC article.
-
Temporal transcriptome profiling in the response to Salmonella enterica serovar enteritidis infection in chicken cecum.Poult Sci. 2025 Feb;104(2):104773. doi: 10.1016/j.psj.2025.104773. Epub 2025 Jan 4. Poult Sci. 2025. PMID: 39813862 Free PMC article.
-
AMPK and mTOR: sensors and regulators of immunometabolic changes during Salmonella infection in the chicken.Poult Sci. 2016 Feb;95(2):345-53. doi: 10.3382/ps/pev349. Epub 2015 Dec 25. Poult Sci. 2016. PMID: 26706353 Review.
Cited by
-
Immunoglobulin secretion influences the composition of chicken caecal microbiota.Sci Rep. 2024 Oct 25;14(1):25410. doi: 10.1038/s41598-024-76856-2. Sci Rep. 2024. PMID: 39455845 Free PMC article.
-
Serum amyloid A regulates TLR2/4-mediated IFN-β signaling pathway against Marek's disease virus.Virus Res. 2023 Mar;326:199044. doi: 10.1016/j.virusres.2023.199044. Epub 2023 Jan 15. Virus Res. 2023. PMID: 36652973 Free PMC article.
-
The early innate response of chickens to Salmonella enterica is dependent on the presence of O-antigen but not on serovar classification.PLoS One. 2014 Apr 24;9(4):e96116. doi: 10.1371/journal.pone.0096116. eCollection 2014. PLoS One. 2014. PMID: 24763249 Free PMC article.
-
Transcriptional responses of cytokines, immunoglobulin A, and nitric oxide genes in 1-day-old chicks post Salmonella typhimurium infection: An experimental study.Open Vet J. 2024 Jan;14(1):200-213. doi: 10.5455/OVJ.2024.v14.i1.18. Epub 2024 Jan 31. Open Vet J. 2024. PMID: 38633162 Free PMC article.
-
Vaccination of chickens with SPI1-lon and SPI1-lon-fliC mutant of Salmonella enterica Serovar Enteritidis.PLoS One. 2013 Jun 13;8(6):e66172. doi: 10.1371/journal.pone.0066172. Print 2013. PLoS One. 2013. PMID: 23785484 Free PMC article.
References
-
- Anonymous. Report of the task force on zoonoses data collection on the analysis of the baseline study on the prevalence of Salmonella in holdings of laying hen flocks of Gallus gallus. EFSA J. 2007;97:1–84.
-
- Lahuerta A, Westrell T, Takkinen J, Boelaert F, Rizzi V, Helwigh B, Borck B, Korsgaard H, Ammon A, Makela P. Zoonoses in the European Union: origin, distribution and dynamics - the EFSA-ECDC summary report 2009. Euro Surveill. 2011;16(13) Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

