CYP4 enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid ω-hydroxylase activities
- PMID: 23688133
- PMCID: PMC4245146
- DOI: 10.2174/15680266113139990110
CYP4 enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid ω-hydroxylase activities
Abstract
The Cytochrome P450 4 (CYP4) family of enzymes in humans is comprised of thirteen isozymes that typically catalyze the ω-oxidation of endogenous fatty acids and eicosanoids. Several CYP4 enzymes can biosynthesize 20- hydroxyeicosatetraenoic acid, or 20-HETE, an important signaling eicosanoid involved in regulation of vascular tone and kidney reabsorption. Additionally, accumulation of certain fatty acids is a hallmark of the rare genetic disorders, Refsum disease and X-ALD. Therefore, modulation of CYP4 enzyme activity, either by inhibition or induction, is a potential strategy for drug discovery. Here we review the substrate specificities, sites of expression, genetic regulation, and inhibition by exogenous chemicals of the human CYP4 enzymes, and discuss the targeting of CYP4 enzymes in the development of new treatments for hypertension, stroke, certain cancers and the fatty acid-linked orphan diseases.
Conflict of interest statement
Figures


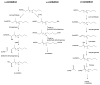
References
-
- Hoch U, Ortiz De Montellano PR. Covalently linked heme in cytochrome p4504a fatty acid hydroxylases. The Journal of biological chemistry. 2001;276(14):11339–11346. - PubMed
-
- Henne KR, Kunze KL, Zheng YM, Christmas P, Soberman RJ, Rettie AE. Covalent linkage of prosthetic heme to CYP4 family P450 enzymes. Biochemistry. 2001;40(43):12925–12931. - PubMed
-
- Bylund J, Hidestrand M, Ingelman-Sundberg M, Oliw EH. Identification of CYP4F8 in human seminal vesicles as a prominent 19-hydroxylase of prostaglandin endoperoxides. The Journal of biological chemistry. 2000;275(29):21844–21849. - PubMed
-
- Bylund J, Bylund M, Oliw EH. cDna cloning and expression of CYP4F12, a novel human cytochrome P450. Biochemical and biophysical research communications. 2001;280(3):892–897. - PubMed
-
- Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O’Donnell CJ, Brown NJ, Waterman MR, Capdevila JH. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111(1):63–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

