Hit identification and optimization in virtual screening: practical recommendations based on a critical literature analysis
- PMID: 23688234
- PMCID: PMC3772997
- DOI: 10.1021/jm301916b
Hit identification and optimization in virtual screening: practical recommendations based on a critical literature analysis
Abstract
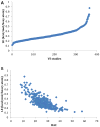
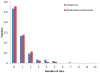
A critical analysis of virtual screening results published between 2007 and 2011 was performed. The activity of reported hit compounds from over 400 studies was compared to their hit identification criteria. Hit rates and ligand efficiencies were calculated to assist in these analyses, and the results were compared with factors such as the size of the virtual library and the number of compounds tested. A series of promiscuity, druglike, and ADMET filters were applied to the reported hits to assess the quality of compounds reported, and a careful analysis of a subset of the studies that presented hit optimization was performed. These data allowed us to make several practical recommendations with respect to selection of compounds for experimental testing, definition of hit identification criteria, and general virtual screening hit criteria to allow for realistic hit optimization. A key recommendation is the use of size-targeted ligand efficiency values as hit identification criteria.
Figures




References
-
- Shun TY, Lazo JS, Sharlow ER, Johnston PA. Identifying actives from HTS data sets: practical approaches for the selection of an appropriate HTS data-processing method and quality control review. J Biomol Screen. 2011;16:1–14. - PubMed
-
- Ripphausen P, Nisius B, Peltason L, Bajorath J. Quo vadis, virtual screening? A comprehensive survey of prospective applications. J Med Chem. 2010;53:8461–8467. - PubMed
-
- Stumpfe D, Ripphausen P, Bajorath J. Virtual compound screening in drug discovery. Future Med Chem. 2012;4:593–602. - PubMed
-
- Ripphausen P, Nisius B, Bajorath J. State-of-the-art in ligand-based virtual screening. Drug Discovery Today. 2011;16:372–376. - PubMed
-
- Scior T, Bender A, Tresadern G, Medina-Franco JL, Martinez-Mayorga K, Langer T, Cuanalo-Contreras K, Agrafiotis DK. Recognizing Pitfalls in Virtual Screening: A Critical Review. J Chem Inf Model. 2012;52:867–881. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

