Treatment of heart failure in adults with thalassemia major: response in patients randomised to deferoxamine with or without deferiprone
- PMID: 23688265
- PMCID: PMC3669105
- DOI: 10.1186/1532-429X-15-38
Treatment of heart failure in adults with thalassemia major: response in patients randomised to deferoxamine with or without deferiprone
Abstract
Background: Established heart failure in thalassaemia major has a poor prognosis and optimal management remains unclear.
Methods: A 1 year prospective study comparing deferoxamine (DFO) monotherapy or when combined with deferiprone (DFP) for patients with left ventricular ejection fraction (LVEF) <56% was conducted by the Thalassemia Clinical Research Network (TCRN). All patients received DFO at 50-60 mg/kg 12-24 hr/day sc or iv 7 times weekly, combined with either DFP 75 at mg/kg/day (combination arm) or placebo (DFO monotherapy arm). The primary endpoint was the change in LVEF by CMR.
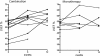
Results: Improvement in LVEF was significant in both study arms at 6 and 12 months (p = 0.04), normalizing ventricular function in 9/16 evaluable patients. With combination therapy, the LVEF increased from 49.9% to 55.2% (+5.3% p = 0.04; n = 10) at 6 months and to 58.3% at 12 months (+8.4% p = 0.04; n = 7). With DFO monotherapy, the LVEF increased from 52.8% to 55.7% (+2.9% p = 0.04; n = 6) at 6 months and to 56.9% at 12 months (+4.1% p = 0.04; n = 4). The LVEF trend did not reach statistical difference between study arms (p = 0.89). In 2 patients on DFO monotherapy during the study and in 1 patient on combined therapy during follow up, heart failure deteriorated fatally. The study was originally powered for 86 participants to determine a 5% difference in LVEF improvement between treatments. The study was prematurely terminated due to slow recruitment and with the achieved sample size of 20 patients there was 80% power to detect an 8.6% difference in EF, which was not demonstrated. Myocardial T2* improved in both arms (combination +1.9 ± 1.6 ms p = 0.04; and DFO monotherapy +1.9 ± 1.4 ms p = 0.04), but with no significant difference between treatments (p = 0.65). Liver iron (p = 0.03) and ferritin (p < 0.001) both decreased significantly in only the combination group.
Conclusions: Both treatments significantly improved LVEF and myocardial T2*. Although this is the largest and only randomized study in patients with LV decompensation, further prospective evaluation is needed to identify optimal chelation management in these high-risk patients.
Figures



Similar articles
-
Cardiac and hepatic iron and ejection fraction in thalassemia major: multicentre prospective comparison of combined deferiprone and deferoxamine therapy against deferiprone or deferoxamine monotherapy.J Cardiovasc Magn Reson. 2013 Jan 16;15(1):1. doi: 10.1186/1532-429X-15-1. J Cardiovasc Magn Reson. 2013. PMID: 23324167 Free PMC article.
-
Effect of deferiprone or deferoxamine on right ventricular function in thalassemia major patients with myocardial iron overload.J Cardiovasc Magn Reson. 2011 Jul 6;13(1):34. doi: 10.1186/1532-429X-13-34. J Cardiovasc Magn Reson. 2011. PMID: 21733147 Free PMC article.
-
Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction.J Cardiovasc Magn Reson. 2008 Feb 25;10(1):12. doi: 10.1186/1532-429X-10-12. J Cardiovasc Magn Reson. 2008. PMID: 18298856 Free PMC article. Clinical Trial.
-
Combined therapy with deferoxamine and deferiprone.Ann N Y Acad Sci. 2005;1054:175-82. doi: 10.1196/annals.1345.020. Ann N Y Acad Sci. 2005. PMID: 16339663 Review.
-
Advances in iron overload therapies. prospects for effective use of deferiprone (L1), deferoxamine, the new experimental chelators ICL670, GT56-252, L1NA11 and their combinations.Curr Med Chem. 2005;12(23):2663-81. doi: 10.2174/092986705774463003. Curr Med Chem. 2005. PMID: 16305464 Review.
Cited by
-
Single-center retrospective study of the effectiveness and toxicity of the oral iron chelating drugs deferiprone and deferasirox.PLoS One. 2019 Feb 27;14(2):e0211942. doi: 10.1371/journal.pone.0211942. eCollection 2019. PLoS One. 2019. PMID: 30811439 Free PMC article.
-
Beyond transfusions and transplants: genomic innovations rewriting the narrative of thalassemia.Ann Hematol. 2025 Aug 16. doi: 10.1007/s00277-025-06548-y. Online ahead of print. Ann Hematol. 2025. PMID: 40817923 Review.
-
Cardiovascular magnetic resonance native T2 and T2* quantitative values for cardiomyopathies and heart transplantations: a systematic review and meta-analysis.J Cardiovasc Magn Reson. 2020 May 11;22(1):34. doi: 10.1186/s12968-020-00627-x. J Cardiovasc Magn Reson. 2020. PMID: 32393281 Free PMC article.
-
Effects of deferasirox-deferoxamine on myocardial and liver iron in patients with severe transfusional iron overload.Blood. 2015 Jun 18;125(25):3868-77. doi: 10.1182/blood-2014-07-586677. Epub 2015 May 1. Blood. 2015. PMID: 25934475 Free PMC article. Clinical Trial.
-
Iron overload in thalassemia: different organs at different rates.Hematology Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):265-271. doi: 10.1182/asheducation-2017.1.265. Hematology Am Soc Hematol Educ Program. 2017. PMID: 29222265 Free PMC article. Review.
References
-
- Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–1236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

