Glycomic analysis using glycoprotein immobilization for glycan extraction
- PMID: 23688297
- PMCID: PMC3696186
- DOI: 10.1021/ac400761e
Glycomic analysis using glycoprotein immobilization for glycan extraction
Abstract
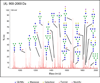
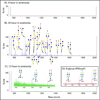
Glycosylation is one of the most common protein modifications and is involved in many functions of glycoproteins. Investigating aberrant protein glycosylation associated with diseases is useful in improving disease diagnostics. Due to the nontemplate nature of glycan biosynthesis, the glycans attached to glycoproteins are enormously complex; thus, a method for comprehensive analysis of glycans from biological or clinical samples is needed. Here, we describe a novel method for glycomic analysis using glycoprotein immobilization for glycan extraction (GIG). Proteins or peptides from complex samples were first immobilized on solid support, and other nonconjugated molecules were removed. Glycans were enzymatically or chemically modified on solid phase before releasing from glycoproteins/glycopeptides for mass spectrometry analysis. The method was applied to the glycomic analysis of both N- and O-glycans.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

