Interferon-α (IFN-α) suppresses HTLV-1 gene expression and cell cycling, while IFN-α combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells
- PMID: 23688327
- PMCID: PMC3698133
- DOI: 10.1186/1742-4690-10-52
Interferon-α (IFN-α) suppresses HTLV-1 gene expression and cell cycling, while IFN-α combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells
Abstract
Background: Human T-cell leukemia virus type-1 (HTLV-1) is the causative retrovirus of adult T-cell leukemia/lymphoma (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). HTLV-1 gene expression is maintained at low levels in vivo by unknown mechanisms. A combination therapy of interferon-α (IFN-α) and zidovudin (AZT) shows therapeutic effects in ATL patients, although its mechanism is also obscure. We previously found that viral gene expression in IL-2-dependent HTLV-1-infected T-cells (ILTs) derived from ATL patients was markedly suppressed by stromal cells through a type I IFN response. Here, we investigated the effects of IFN-α with or without AZT on viral gene expression and cell growth in ILTs.
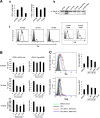
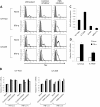
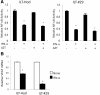
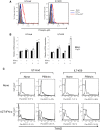
Results: ILTs expressed variable but lower amounts of HTLV-1 Tax protein than HTLV-1-transformed HUT102 cells. Following the addition of IFN-α, the amounts of HTLV-1 p19 in the supernatants of these cells decreased in three days, while HTLV-1 gene expression decreased only in ILTs but not HUT102 cells. IFN-α also suppressed the spontaneous HTLV-1 induction in primary ATL cells cultured for 24 h. A time course study using ILTs revealed that the levels of intracellular Tax proteins decreased in the first 24 h after addition of IFN-α, before the reduction in HTLV-1 mRNA levels. The initial decreases of Tax protein following IFN-α treatment were observed in 6 of 7 ILT lines tested, although the reduction rates varied among ILT lines. An RNA-dependent protein kinase (PKR)-inhibitor reversed IFN-mediated suppression of Tax in ILTs. IFN-α also induced cell cycle arrest at the G0/G1 phase and suppressed NF-κB activities in these cells. AZT alone did not affect HTLV-1 gene expression, cell viability or NF-κB activities. AZT combined with IFN-α markedly induced cell apoptosis associated with phosphorylation of p53 and induction of p53-responsive genes in ILTs.
Conclusions: IFN-α suppressed HTLV-1 gene expression at least through a PKR-mediated mechanism, and also induced cell cycle arrest in ILTs. In combination with AZT, IFN-α further induced p53 signaling and cell apoptosis in these cells. These findings suggest that HTLV-1-infected cells at an IL-2-dependent stage retain susceptibility to type I IFN-mediated regulation of viral expression, and partly explain how AZT/IFN-α produces therapeutic effects in ATL.
Figures


Similar articles
-
Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation.J Virol. 2016 Mar 28;90(8):3902-3912. doi: 10.1128/JVI.00129-16. Print 2016 Apr. J Virol. 2016. PMID: 26819312 Free PMC article.
-
IL-10-mediated signals act as a switch for lymphoproliferation in Human T-cell leukemia virus type-1 infection by activating the STAT3 and IRF4 pathways.PLoS Pathog. 2017 Sep 14;13(9):e1006597. doi: 10.1371/journal.ppat.1006597. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28910419 Free PMC article.
-
Stromal cell-mediated suppression of human T-cell leukemia virus type 1 expression in vitro and in vivo by type I interferon.J Virol. 2009 May;83(10):5101-8. doi: 10.1128/JVI.02564-08. Epub 2009 Mar 4. J Virol. 2009. PMID: 19264779 Free PMC article.
-
Impact of host immunity on HTLV-1 pathogenesis: potential of Tax-targeted immunotherapy against ATL.Retrovirology. 2019 Aug 22;16(1):23. doi: 10.1186/s12977-019-0484-z. Retrovirology. 2019. PMID: 31438973 Free PMC article. Review.
-
HTLV-1 Tax and adult T-cell leukemia.Front Biosci. 2007 Jan 1;12:1496-507. doi: 10.2741/2163. Front Biosci. 2007. PMID: 17127397 Review.
Cited by
-
Cabotegravir, the Long-Acting Integrase Strand Transfer Inhibitor, Potently Inhibits Human T-Cell Lymphotropic Virus Type 1 Transmission in vitro.Front Med (Lausanne). 2022 Apr 25;9:889621. doi: 10.3389/fmed.2022.889621. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35547224 Free PMC article.
-
Comprehensive Antiretroviral Restriction Factor Profiling Reveals the Evolutionary Imprint of the ex Vivo and in Vivo IFN-β Response in HTLV-1-Associated Neuroinflammation.Front Microbiol. 2018 May 22;9:985. doi: 10.3389/fmicb.2018.00985. eCollection 2018. Front Microbiol. 2018. PMID: 29872426 Free PMC article.
-
Naringin Reverses Hepatocyte Apoptosis and Oxidative Stress Associated with HIV-1 Nucleotide Reverse Transcriptase Inhibitors-Induced Metabolic Complications.Nutrients. 2015 Dec 10;7(12):10352-68. doi: 10.3390/nu7125540. Nutrients. 2015. PMID: 26690471 Free PMC article.
-
Molecular Studies of HTLV-1 Replication: An Update.Viruses. 2016 Jan 27;8(2):31. doi: 10.3390/v8020031. Viruses. 2016. PMID: 26828513 Free PMC article. Review.
-
Interplay between innate immunity and the viral oncoproteins Tax and HBZ in the pathogenesis and therapeutic response of HTLV-1 associated adult T cell leukemia.Front Immunol. 2022 Jul 22;13:957535. doi: 10.3389/fimmu.2022.957535. eCollection 2022. Front Immunol. 2022. PMID: 35935975 Free PMC article. Review.
References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. - PubMed
-
- Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. - PubMed
-
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

