Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production
- PMID: 23688412
- PMCID: PMC3704056
- DOI: 10.1016/j.jaci.2013.03.048
Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production
Abstract
Background: Cysteinyl leukotrienes (CysLTs) contribute to asthma pathogenesis, in part through cysteinyl leukotriene receptor 1 (CysLT1R). Recently discovered lineage-negative type 2 innate lymphoid cells (ILC2s) potently produce IL-5 and IL-13.
Objectives: We hypothesized that lung ILC2s might be activated by leukotrienes through CysLT1R.
Methods: ILC2s (Thy1.2(+) lineage-negative lymphocytes) and CysLT1R were detected in the lungs of wild-type, signal transducer and activator of transcription 6-deficient (STAT6(-/-)), and recombination-activating gene 2-deficient (RAG2(-/-)) mice by means of flow cytometry. T(H)2 cytokine levels were measured in purified lung ILC2s stimulated with leukotriene D₄ (LTD₄) in the presence or absence of the CysLT1R antagonist montelukast. Calcium influx was measured by using Fluo-4 intensity. Intranasal leukotriene C₄, D₄, and E₄ were administered to naive mice, and levels of ILC2 IL-5 production were determined. Finally, LTD₄ was coadministered with Alternaria species repetitively to RAG2(-/-) mice (with ILC2s) and IL-7 receptor-deficient mice (lack ILC2s), and total ILC2 numbers, proliferation (Ki-67(+)), and bronchoalveolar lavage fluid eosinophil numbers were measured.
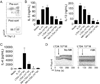
Results: CysLT1R was expressed on lung ILC2s from wild-type, RAG2(-/-), and STAT6(-/-) naive and Alternaria species-challenged mice. In vitro LTD₄ induced ILC2s to rapidly generate high levels of IL-5 and IL-13 within 6 hours of stimulation. Interestingly, LTD4, but not IL-33, induced high levels of IL-4 by ILC2s. LTD₄ administered in vivo rapidly induced ILC2 IL-5 production that was significantly reduced by montelukast before treatment. Finally, LTD₄ potentiated Alternaria species-induced eosinophilia, as well as ILC2 accumulation and proliferation.
Conclusions: We present novel data that CysLT1R is expressed on ILC2s and LTD₄ potently induces CysLT1R-dependent ILC2 production of IL-4, IL-5, and IL-13. Additionally, LTD₄ potentiates Alternaria species-induced eosinophilia and ILC2 proliferation and accumulation.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures




Comment in
-
Activation of group 2 innate lymphoid cells: a new role for cysteinyl leukotrienes.J Allergy Clin Immunol. 2013 Jul;132(1):214-6. doi: 10.1016/j.jaci.2013.05.019. J Allergy Clin Immunol. 2013. PMID: 23810099 Free PMC article. No abstract available.
References
-
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. - PubMed
-
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

