Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults
- PMID: 23688546
- PMCID: PMC3668902
- DOI: 10.1186/1471-2334-13-224
Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults
Abstract
Background: Two phylogenetic lineages of influenza B virus coexist and circulate in the human population (B/Yamagata and B/Victoria) but only one B-strain is included in each seasonal vaccine. Mismatch regularly occurs between the recommended and circulating B-strain. Inclusion of both lineages in vaccines may offer better protection against influenza.
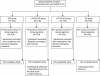
Methods: This study (NCT00714285) assessed the immunogenicity and safety of two candidate quadrivalent influenza vaccines (QIV) containing two A- and two B-strains (one from each lineage) in adults (18-60 years). Subjects were randomized and stratified by age to receive either QIV (non-adjuvanted or low-dose adjuvanted [LD QIV-AS]) or trivalent influenza vaccine (TIV, non-adjuvanted or low-dose adjuvanted [LD TIV-AS]), N = 105 in all treatment groups. The study evaluated the statistical non-inferiority of the immunological response elicited by QIV and LD QIV-AS versus TIV and LD TIV-AS and the statistical superiority of the response elicited by the quadrivalent vaccines against the B-strain (B/Jiangsu) not included in the TIV.
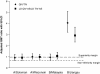
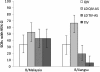
Results: Pre-defined non-inferiority and superiority criteria were reached for both QIVs compared to the TIVs. On Day 21 in all vaccine groups SCRs were ≥54.8%, SPRs ≥88.5% and SCFs ≥5.4 for the A strains and B strain included in all vaccines (B/Malaysia). This fulfilled the European (CHMP) and the US (CBER) licensing criteria for the assessment of influenza vaccines in adults (CHMP criteria: SCR > 40%, SPR > 70%, SCF > 2; CBER criteria: LL of 95% CI for SPR ≥ 70% or SCR ≥ 40%). Only the QIVs met the CHMP and CBER criteria for the B/Jiangsu strain. In the QIV and LD-QIV-AS groups, the SCFs were 9.1 and 8.1, respectively and the SPRs were 98.1% and 95.2%, whereas for the TIV and LD-TIV-AS groups, the SCFs were 2.3 and 2.5, respectively, and the SPRs were 75.0% and 63.8%, with the LLs of the 95% CI <70% for SPR and <40% for SCR.
Conclusions: Addition of a fourth strain did not impact the immune response elicited by the three original strains contained in the TIV. A clear immunological benefit was seen with the QIV formulation for the second B-strain, indicating that quadrivalent vaccines could provide broader protection against influenza.
Trial registration: ClinicalTrials.gov: NCT00714285.
Figures
References
-
- Barr IG, Komadina N, Durrant C, Sjogren H, Hurt AC, Shaw RP. Circulation and antigenic drift in human influenza B viruses in SE Asia and Oceania since 2000. Commun Dis Intell. 2006;30:350–357. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

