Phase 1 study of dose escalation in hypofractionated proton beam therapy for non-small cell lung cancer
- PMID: 23688815
- PMCID: PMC3926094
- DOI: 10.1016/j.ijrobp.2013.03.035
Phase 1 study of dose escalation in hypofractionated proton beam therapy for non-small cell lung cancer
Abstract
Background: Many patients with locally advanced non-small cell lung cancer (NSCLC) cannot undergo concurrent chemotherapy because of comorbidities or poor performance status. Hypofractionated radiation regimens, if tolerable, may provide an option to these patients for effective local control.
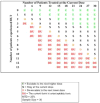
Methods and materials: Twenty-five patients were enrolled in a phase 1 dose-escalation trial of proton beam therapy (PBT) from September 2010 through July 2012. Eligible patients had histologically documented lung cancer, thymic tumors, carcinoid tumors, or metastatic thyroid tumors. Concurrent chemotherapy was not allowed, but concurrent treatment with biologic agents was. The dose-escalation schema comprised 15 fractions of 3 Gy(relative biological effectiveness [RBE])/fraction, 3.5 Gy(RBE)/fraction, or 4 Gy(RBE)/fraction. Dose constraints were derived from biologically equivalent doses of standard fractionated treatment.
Results: The median follow-up time for patients alive at the time of analysis was 13 months (range, 8-28 months). Fifteen patients received treatment to hilar or mediastinal lymph nodes. Two patients experienced dose-limiting toxicity possibly related to treatment; 1 received 3.5-Gy(RBE) fractions and experienced an in-field tracheoesophageal fistula 9 months after PBT and 1 month after bevacizumab. The other patient received 4-Gy(RBE) fractions and was hospitalized for bacterial pneumonia/radiation pneumonitis 4 months after PBT.
Conclusion: Hypofractionated PBT to the thorax delivered over 3 weeks was well tolerated even with significant doses to the lungs and mediastinal structures. Phase 2/3 trials are needed to compare the efficacy of this technique with standard treatment for locally advanced NSCLC.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002;94:173–181. - PubMed
-
- Belani CP, Fossella F. Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326) Cancer. 2005;104:2766–2774. - PubMed
-
- Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer. 2001;92:2639–2647. - PubMed
-
- Nguyen LN, Komaki R, Allen P, et al. Effectiveness of accelerated radiotherapy for patients with inoperable non-small cell lung cancer (NSCLC) and borderline prognostic factors without distant metastasis: a retrospective review. Int J Radiat Oncol Biol Phys. 1999;44:1053–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

