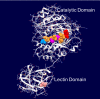
The lectin domain of the polypeptide GalNAc transferase family of glycosyltransferases (ppGalNAc Ts) acts as a switch directing glycopeptide substrate glycosylation in an N- or C-terminal direction, further controlling mucin type O-glycosylation
- PMID: 23689369
- PMCID: PMC3707691
- DOI: 10.1074/jbc.M113.477877
The lectin domain of the polypeptide GalNAc transferase family of glycosyltransferases (ppGalNAc Ts) acts as a switch directing glycopeptide substrate glycosylation in an N- or C-terminal direction, further controlling mucin type O-glycosylation
Abstract
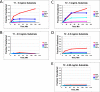
Mucin type O-glycosylation is initiated by a large family of polypeptide GalNAc transferases (ppGalNAc Ts) that add α-GalNAc to the Ser and Thr residues of peptides. Of the 20 human isoforms, all but one are composed of two globular domains linked by a short flexible linker: a catalytic domain and a ricin-like lectin carbohydrate binding domain. Presently, the roles of the catalytic and lectin domains in peptide and glycopeptide recognition and specificity remain unclear. To systematically study the role of the lectin domain in ppGalNAc T glycopeptide substrate utilization, we have developed a series of novel random glycopeptide substrates containing a single GalNAc-O-Thr residue placed near either the N or C terminus of the glycopeptide substrate. Our results reveal that the presence and N- or C-terminal placement of the GalNAc-O-Thr can be important determinants of overall catalytic activity and specificity that differ between transferase isoforms. For example, ppGalNAc T1, T2, and T14 prefer C-terminally placed GalNAc-O-Thr, whereas ppGalNAc T3 and T6 prefer N-terminally placed GalNAc-O-Thr. Several transferase isoforms, ppGalNAc T5, T13, and T16, display equally enhanced N- or C-terminal activities relative to the nonglycosylated control peptides. This N- and/or C-terminal selectivity is presumably due to weak glycopeptide binding to the lectin domain, whose orientation relative to the catalytic domain is dynamic and isoform-dependent. Such N- or C-terminal glycopeptide selectivity provides an additional level of control or fidelity for the O-glycosylation of biologically significant sites and suggests that O-glycosylation may in some instances be exquisitely controlled.
Keywords: Control of Glycosylation; Convertases; Edman Sequencing; Glycobiology; Glycoprotein Biosynthesis; Glycosyltransferases; Lectin; Mucins; O-Glycosylation; Random Glycopeptide.
Figures





Similar articles
-
Mucin-type O-glycosylation is controlled by short- and long-range glycopeptide substrate recognition that varies among members of the polypeptide GalNAc transferase family.Glycobiology. 2016 Apr;26(4):360-76. doi: 10.1093/glycob/cwv108. Epub 2015 Nov 26. Glycobiology. 2016. PMID: 26610890 Free PMC article.
-
Glycopeptide-preferring polypeptide GalNAc transferase 10 (ppGalNAc T10), involved in mucin-type O-glycosylation, has a unique GalNAc-O-Ser/Thr-binding site in its catalytic domain not found in ppGalNAc T1 or T2.J Biol Chem. 2009 Jul 24;284(30):20387-97. doi: 10.1074/jbc.M109.017236. Epub 2009 May 21. J Biol Chem. 2009. PMID: 19460755 Free PMC article.
-
Lectin domains of polypeptide GalNAc transferases exhibit glycopeptide binding specificity.J Biol Chem. 2011 Sep 16;286(37):32684-96. doi: 10.1074/jbc.M111.273722. Epub 2011 Jul 15. J Biol Chem. 2011. PMID: 21768105 Free PMC article.
-
Polypeptide GalNAc-Ts: from redundancy to specificity.Curr Opin Struct Biol. 2019 Jun;56:87-96. doi: 10.1016/j.sbi.2018.12.007. Epub 2019 Jan 28. Curr Opin Struct Biol. 2019. PMID: 30703750 Free PMC article. Review.
-
Molecular Recognition of GalNAc in Mucin-Type O-Glycosylation.Acc Chem Res. 2023 Mar 7;56(5):548-560. doi: 10.1021/acs.accounts.2c00723. Epub 2023 Feb 23. Acc Chem Res. 2023. PMID: 36815693 Free PMC article. Review.
Cited by
-
Polypeptide N-Acetylgalactosaminyltransferase 13 Contributes to Neurogenesis via Stabilizing the Mucin-type O-Glycoprotein Podoplanin.J Biol Chem. 2016 Nov 4;291(45):23477-23488. doi: 10.1074/jbc.M116.743955. Epub 2016 Sep 14. J Biol Chem. 2016. PMID: 27629416 Free PMC article.
-
Structural and Mechanistic Insights into the Catalytic-Domain-Mediated Short-Range Glycosylation Preferences of GalNAc-T4.ACS Cent Sci. 2018 Sep 26;4(9):1274-1290. doi: 10.1021/acscentsci.8b00488. Epub 2018 Sep 14. ACS Cent Sci. 2018. PMID: 30276263 Free PMC article.
-
Molecular basis for fibroblast growth factor 23 O-glycosylation by GalNAc-T3.Nat Chem Biol. 2020 Mar;16(3):351-360. doi: 10.1038/s41589-019-0444-x. Epub 2020 Jan 13. Nat Chem Biol. 2020. PMID: 31932717 Free PMC article.
-
Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis.Glycobiology. 2015 Jan;25(1):55-65. doi: 10.1093/glycob/cwu089. Epub 2014 Aug 25. Glycobiology. 2015. PMID: 25155433 Free PMC article.
-
Exploring Regulation of Protein O-Glycosylation in Isogenic Human HEK293 Cells by Differential O-Glycoproteomics.Mol Cell Proteomics. 2019 Jul;18(7):1396-1409. doi: 10.1074/mcp.RA118.001121. Epub 2019 Apr 30. Mol Cell Proteomics. 2019. PMID: 31040225 Free PMC article.
References
-
- Hollingsworth M. A., Swanson B. J. (2004) Mucins in cancer. Protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60 - PubMed
-
- Gill D. J., Clausen H., Bard F. (2011) Location, location, location. New insights into O-GalNAc protein glycosylation. Trends Cell Biol. 21, 149–158 - PubMed
-
- Wopereis S., Lefeber D. J., Morava E., Wevers R. A. (2006) Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects. A Review. Clin. Chem. 52, 574–600 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

