Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy
- PMID: 23689599
- PMCID: PMC3702100
- DOI: 10.1038/mt.2013.86
Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy
Abstract
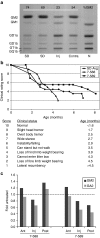
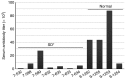
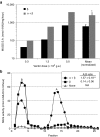
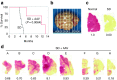
Salutary responses to adeno-associated viral (AAV) gene therapy have been reported in the mouse model of Sandhoff disease (SD), a neurodegenerative lysosomal storage disease caused by deficiency of β-N-acetylhexosaminidase (Hex). While untreated mice reach the humane endpoint by 4.1 months of age, mice treated by a single intracranial injection of vectors expressing human hexosaminidase may live a normal life span of 2 years. When treated with the same therapeutic vectors used in mice, two cats with SD lived to 7.0 and 8.2 months of age, compared with an untreated life span of 4.5 ± 0.5 months (n = 11). Because a pronounced humoral immune response to both the AAV1 vectors and human hexosaminidase was documented, feline cDNAs for the hexosaminidase α- and β-subunits were cloned into AAVrh8 vectors. Cats treated with vectors expressing feline hexosaminidase produced enzymatic activity >75-fold normal at the brain injection site with little evidence of an immune infiltrate. Affected cats treated with feline-specific vectors by bilateral injection of the thalamus lived to 10.4 ± 3.7 months of age (n = 3), or 2.3 times as long as untreated cats. These studies support the therapeutic potential of AAV vectors for SD and underscore the importance of species-specific cDNAs for translational research.
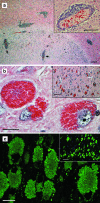
Figures
References
-
- Gravel RA, Clarke JTR, Kaback MM, Mahuran D, Sandhoff K, Suzuki K.1995The GM2 gangliosidoses. Scriver CR, Beaudet AL, Sly WS.Valle D.eds). The Metabolic and Molecular Bases of Inherited Disease7th edn., vol. 2McGraw-Hill, Inc.New York; pp. 2839–2879.
-
- Tay W. Symmetrical changes in the region of the yellow spot in each eye of an infant. Tran Ophthalmol Soc UK. 1881;1:155–157.
-
- Arfi A, Bourgoin C, Basso L, Emiliani C, Tancini B, Chigorno V, et al. Bicistronic lentiviral vector corrects beta-hexosaminidase deficiency in transduced and cross-corrected human Sandhoff fibroblasts. Neurobiol Dis. 2005;20:583–593. - PubMed
-
- Guidotti JE, Mignon A, Haase G, Caillaud C, McDonell N, Kahn A, et al. Adenoviral gene therapy of the Tay-Sachs disease in hexosaminidase A-deficient knock-out mice. Hum Mol Genet. 1999;8:831–838. - PubMed
-
- Itakura T, Kuroki A, Ishibashi Y, Tsuji D, Kawashita E, Higashine Y, et al. Inefficiency in GM2 ganglioside elimination by human lysosomal beta-hexosaminidase beta-subunit gene transfer to fibroblastic cell line derived from Sandhoff disease model mice. Biol Pharm Bull. 2006;29:1564–1569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

