Receptor-independent effects of 2'(3')-O-(4-benzoylbenzoyl)ATP triethylammonium salt on cytosolic pH
- PMID: 23689980
- PMCID: PMC3889390
- DOI: 10.1007/s11302-013-9365-4
Receptor-independent effects of 2'(3')-O-(4-benzoylbenzoyl)ATP triethylammonium salt on cytosolic pH
Abstract
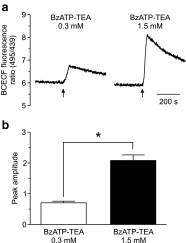
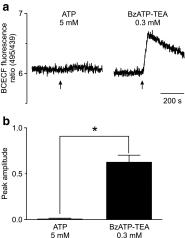
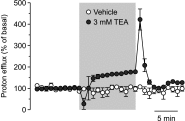
The effect of the relatively potent P2X7 receptor agonist 2'(3')-O-(4-benzoylbenzoyl)adenosine 5'-triphosphate triethylammonium salt (BzATP-TEA) on cytosolic pH (pHi) was studied using MC3T3-E1 osteoblast-like cells, which endogenously express P2X7 receptors. pHi was measured fluorimetrically using the pH-sensitive dye 2',7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein. BzATP-TEA (0.3-1.5 mM) elicited fast-onset alkalinization responses. In contrast, adenosine 5'-triphosphate disodium salt (5 mM) failed to reproduce the BzATP-TEA-induced responses, indicating a P2 receptor-independent mechanism. We speculated that triethylamine, which is present in solutions of BzATP-TEA, permeates the plasma membrane, and is protonated intracellularly, leading to an increase in pHi. Consistent with this hypothesis, triethylammonium (TEA) chloride mimicked the effects of BzATP-TEA on pHi. Moreover, measurements using a Cytosensor microphysiometer revealed that TEA chloride transiently suppressed proton efflux from cells, whereas washout of TEA transiently enhanced proton efflux. BzATP-TEA also elicited a sustained increase in proton efflux that was blocked specifically by the P2X7 antagonist A-438079. Taken together, we conclude that BzATP-TEA-induced alkalinization is unrelated to P2X7 activation, but is due to the presence of TEA. This effect may confound assessment of the outcomes of P2X7 activation by BzATP-TEA in other systems. Thus, control experiments using TEA chloride are recommended to distinguish between receptor-mediated and nonspecific effects of this widely used agonist. We performed such a control and confirmed that BzATP-TEA, but not TEA chloride, caused the elevation of cytosolic free Ca(2+) in MC3T3-E1 cells, ruling out the possibility that receptor-independent effects on pHi underlie BzATP-TEA-induced Ca(2+) signaling.
Figures





Similar articles
-
P2X₇-mediated calcium influx triggers a sustained, PI3K-dependent increase in metabolic acid production by osteoblast-like cells.Am J Physiol Endocrinol Metab. 2012 Mar 1;302(5):E561-75. doi: 10.1152/ajpendo.00209.2011. Epub 2011 Dec 20. Am J Physiol Endocrinol Metab. 2012. PMID: 22185840
-
Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2'-3'-O-(4-benzoylbenzoyl)-ATP.J Neurosci. 2004 Aug 11;24(32):7128-39. doi: 10.1523/JNEUROSCI.2093-04.2004. J Neurosci. 2004. PMID: 15306646 Free PMC article.
-
P2X7 receptor activation downmodulates Na(+)-dependent high-affinity GABA and glutamate transport into rat brain cortex synaptosomes.Neuroscience. 2015 Oct 15;306:74-90. doi: 10.1016/j.neuroscience.2015.08.026. Epub 2015 Aug 20. Neuroscience. 2015. PMID: 26299340
-
Peripheral P2X7 receptor-induced mechanical hyperalgesia is mediated by bradykinin.Neuroscience. 2014 Sep 26;277:163-73. doi: 10.1016/j.neuroscience.2014.06.057. Epub 2014 Jul 2. Neuroscience. 2014. PMID: 24997266
-
Cholinergic agonists activate P2X7 receptors to stimulate protein secretion by the rat lacrimal gland.Invest Ophthalmol Vis Sci. 2011 May 1;52(6):3381-90. doi: 10.1167/iovs.11-7210. Invest Ophthalmol Vis Sci. 2011. PMID: 21421880 Free PMC article.
Cited by
-
P2X7 ionotropic receptor is functionally expressed in rabbit articular chondrocytes and mediates extracellular ATP cytotoxicity.Purinergic Signal. 2018 Sep;14(3):245-258. doi: 10.1007/s11302-018-9611-x. Epub 2018 May 29. Purinergic Signal. 2018. PMID: 29845461 Free PMC article.
References
-
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. - PubMed
-
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/S0014-2999(99)00350-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

