Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation
- PMID: 23690441
- PMCID: PMC3674702
- DOI: 10.1084/jem.20121588
Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation
Abstract
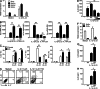
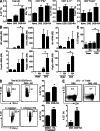
Retinoic acid (RA), a vitamin A metabolite, modulates mucosal T helper cell responses. Here we examined the role of RA in regulating IL-22 production by γδ T cells and innate lymphoid cells in intestinal inflammation. RA significantly enhanced IL-22 production by γδ T cells stimulated in vitro with IL-1β or IL-18 and IL-23. In vivo RA attenuated colon inflammation induced by dextran sodium sulfate treatment or Citrobacter rodentium infection. This was associated with a significant increase in IL-22 secretion by γδ T cells and innate lymphoid cells. In addition, RA treatment enhanced production of the IL-22-responsive antimicrobial peptides Reg3β and Reg3γ in the colon. The attenuating effects of RA on colitis were reversed by treatment with an anti-IL-22 neutralizing antibody, demonstrating that RA mediates protection by enhancing IL-22 production. To define the molecular events involved, we used chromatin immunoprecipitation assays and found that RA promoted binding of RA receptor to the IL-22 promoter in γδ T cells. Our findings provide novel insights into the molecular events controlling IL-22 transcription and suggest that one key outcome of RA signaling may be to shape early intestinal immune responses by promoting IL-22 synthesis by γδ T cells and innate lymphoid cells.
Figures



References
-
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

