Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression
- PMID: 23690473
- PMCID: PMC3679246
- DOI: 10.4049/jimmunol.1202830
Mutated PPP1R3B is recognized by T cells used to treat a melanoma patient who experienced a durable complete tumor regression
Abstract
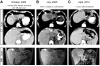
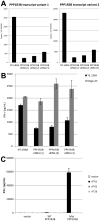
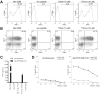
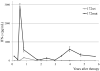
Adoptive cell therapy with tumor-infiltrating lymphocytes (TILs) represents an effective treatment for patients with metastatic melanoma. However, most of the Ag targets recognized by effective melanoma-reactive TILs remain elusive. In this study, patient 2369 experienced a complete response, including regressions of bulky liver tumor masses, ongoing beyond 7 y following adoptive TIL transfer. The screening of a cDNA library generated from the autologous melanoma cell line resulted in the isolation of a mutated protein phosphatase 1, regulatory (inhibitor) subunit 3B (PPP1R3B) gene product. The mutated PPP1R3B peptide represents the immunodominant epitope recognized by tumor-reactive T cells in TIL 2369. Five years following adoptive transfer, peripheral blood T lymphocytes obtained from patient 2369 recognized the mutated PPP1R3B epitope. These results demonstrate that adoptive T cell therapy targeting a tumor-specific Ag can mediate long-term survival for a patient with metastatic melanoma. This study also provides an impetus to develop personalized immunotherapy targeting tumor-specific, mutated Ags.
Figures



References
-
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr., Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. - PMC - PubMed
-
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. - PMC - PubMed
-
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

