Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE)
- PMID: 23690531
- PMCID: PMC3714493
- DOI: 10.2337/dc13-0356
Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE)
Erratum in
-
Erratum. Rationale and Design of the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE). Diabetes Care 2013;36:2254-2261.Diabetes Care. 2022 Mar 1;45(3):759. doi: 10.2337/dc22-er03a. Diabetes Care. 2022. PMID: 35020815 Free PMC article. No abstract available.
Abstract
Objective: The epidemic of type 2 diabetes (T2DM) threatens to become the major public health problem of this century. However, a comprehensive comparison of the long-term effects of medications to treat T2DM has not been conducted. GRADE, a pragmatic, unmasked clinical trial, aims to compare commonly used diabetes medications, when combined with metformin, on glycemia-lowering effectiveness and patient-centered outcomes.
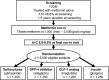
Research design and methods: GRADE was designed with support from a U34 planning grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The consensus protocol was approved by NIDDK and the GRADE Research Group. Eligibility criteria for the 5,000 metformin-treated subjects include <5 years' diabetes duration, ≥ 30 years of age at time of diagnosis, and baseline hemoglobin A1c (A1C) of 6.8-8.5% (51-69 mmol/mol). Medications representing four classes (sulfonylureas, dipeptidyl peptidase 4 inhibitors, glucagon-like peptide 1 receptor agonists, and insulin) will be randomly assigned and added to metformin (minimum-maximum 1,000-2,000 mg/day). The primary metabolic outcome is the time to primary failure defined as an A1C ≥ 7% (53 mmol/mol), subsequently confirmed, over an anticipated mean observation period of 4.8 years (range 4-7 years). Other long-term metabolic outcomes include the need for the addition of basal insulin after a confirmed A1C >7.5% (58 mmol/mol) and, ultimately, the need to implement an intensive basal/bolus insulin regimen. The four drugs will also be compared with respect to selected microvascular complications, cardiovascular disease risk factors, adverse effects, tolerability, quality of life, and cost-effectiveness.
Conclusions: GRADE will compare the long-term effectiveness of major glycemia-lowering medications and provide guidance to clinicians about the most appropriate medications to treat T2DM. GRADE begins recruitment at 37 centers in the U.S. in 2013.
Figures
Comment in
-
Comparative effectiveness and the future of clinical research in diabetes.Diabetes Care. 2013 Aug;36(8):2146-7. doi: 10.2337/dc13-1221. Diabetes Care. 2013. PMID: 23881966 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention. National diabetes fact sheet, 2011 [Internet]. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed 25 January 2013
-
- Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med 1993;328:1676–1685 - PubMed
-
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [article online], 2013. Available from http://care.diabetesjournals.org/content/early/2013/03/05/dc12-2625.full.... Accessed 8 May 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical