The iodide-transport-defect-causing mutation R124H: a δ-amino group at position 124 is critical for maturation and trafficking of the Na+/I- symporter
- PMID: 23690546
- PMCID: PMC3730242
- DOI: 10.1242/jcs.120246
The iodide-transport-defect-causing mutation R124H: a δ-amino group at position 124 is critical for maturation and trafficking of the Na+/I- symporter
Abstract
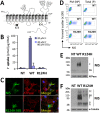
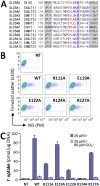
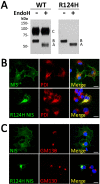
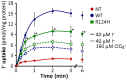
Na(+)/I(-) symporter (NIS)-mediated active accumulation of I(-) in thyrocytes is a key step in the biosynthesis of the iodine-containing thyroid hormones T3 and T4. Several NIS mutants have been identified as a cause of congenital I(-) transport defect (ITD), and their investigation has yielded valuable mechanistic information on NIS. Here we report novel findings derived from the thorough characterization of the ITD-causing mutation R124H, located in the second intracellular loop (IL-2). R124H NIS is incompletely glycosylated and colocalizes with endoplasmic reticulum (ER)-resident protein markers. As a result, R124H NIS is not targeted to the plasma membrane and therefore does not mediate any I(-) transport in transfected COS-7 cells. Strikingly, however, the mutant is intrinsically active, as revealed by its ability to mediate I(-) transport in membrane vesicles. Of all the amino acid substitutions we carried out at position 124 (K, D, E, A, W, N and Q), only Gln restored targeting of NIS to the plasma membrane and NIS activity, suggesting a key structural role for the δ-amino group of R124 in the transporter's maturation and cell surface targeting. Using our NIS homology model based on the structure of the Vibrio parahaemolyticus Na(+)/galactose symporter, we propose an interaction between the δ-amino group of either R or Q124 and the thiol group of C440, located in IL-6. We conclude that the interaction between IL-2 and IL-6 is critical for the local folding required for NIS maturation and plasma membrane trafficking.
Keywords: Congenital iodide transport defect; Impaired intracellular trafficking; Membrane vesicles; Na+/I- symporter (NIS); Plasma membrane targeting.
Figures



References
-
- Danilov S. M., Kalinin S., Chen Z., Vinokour E. I., Nesterovitch A. B., Schwartz D. E., Gribouval O., Gubler M. C., Minshall R. D. (2010). Angiotensin I-converting enzyme Gln1069Arg mutation impairs trafficking to the cell surface resulting in selective denaturation of the C-domain. PLoS ONE 5, e10438 10.1371/journal.pone.0010438 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

