Bacteriophage adhering to mucus provide a non-host-derived immunity
- PMID: 23690590
- PMCID: PMC3696810
- DOI: 10.1073/pnas.1305923110
Bacteriophage adhering to mucus provide a non-host-derived immunity
Abstract
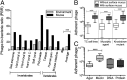
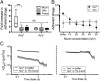
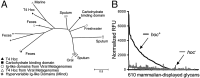
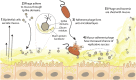
Mucosal surfaces are a main entry point for pathogens and the principal sites of defense against infection. Both bacteria and phage are associated with this mucus. Here we show that phage-to-bacteria ratios were increased, relative to the adjacent environment, on all mucosal surfaces sampled, ranging from cnidarians to humans. In vitro studies of tissue culture cells with and without surface mucus demonstrated that this increase in phage abundance is mucus dependent and protects the underlying epithelium from bacterial infection. Enrichment of phage in mucus occurs via binding interactions between mucin glycoproteins and Ig-like protein domains exposed on phage capsids. In particular, phage Ig-like domains bind variable glycan residues that coat the mucin glycoprotein component of mucus. Metagenomic analysis found these Ig-like proteins present in the phages sampled from many environments, particularly from locations adjacent to mucosal surfaces. Based on these observations, we present the bacteriophage adherence to mucus model that provides a ubiquitous, but non-host-derived, immunity applicable to mucosal surfaces. The model suggests that metazoan mucosal surfaces and phage coevolve to maintain phage adherence. This benefits the metazoan host by limiting mucosal bacteria, and benefits the phage through more frequent interactions with bacterial hosts. The relationships shown here suggest a symbiotic relationship between phage and metazoan hosts that provides a previously unrecognized antimicrobial defense that actively protects mucosal surfaces.
Keywords: host-pathogen; immune system; immunoglobulin; symbiosis; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Sticky bacteriophage protect animal cells.Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10475-6. doi: 10.1073/pnas.1307782110. Epub 2013 Jun 7. Proc Natl Acad Sci U S A. 2013. PMID: 23749871 Free PMC article. No abstract available.
-
Phage biology: A new barrier at mucosal surfaces.Nat Rev Microbiol. 2013 Jul;11(7):430-1. doi: 10.1038/nrmicro3064. Nat Rev Microbiol. 2013. PMID: 23765077 No abstract available.
-
Bacteriophage—Protectors at Mucosal Surfaces.Clin Infect Dis. 2013 Oct;57(8):iv. Clin Infect Dis. 2013. PMID: 24195118 No abstract available.
References
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. - PubMed
-
- Clay K, Holah J. Fungal endophyte symbiosis and plant diversity in successional fields. Science. 1999;285(5434):1742–1745. - PubMed
-
- Douglas AE. Mycetocyte symbiosis in insects. Biol Rev Camb Philos Soc. 1989;64(4):409–434. - PubMed
-
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22(1):283–307. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

