Brain homogenates from human tauopathies induce tau inclusions in mouse brain
- PMID: 23690619
- PMCID: PMC3677441
- DOI: 10.1073/pnas.1301175110
Brain homogenates from human tauopathies induce tau inclusions in mouse brain
Abstract
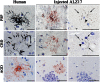
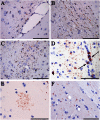
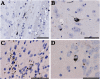
Filamentous inclusions made of hyperphosphorylated tau are characteristic of numerous human neurodegenerative diseases, including Alzheimer's disease, tangle-only dementia, Pick disease, argyrophilic grain disease (AGD), progressive supranuclear palsy, and corticobasal degeneration. In Alzheimer's disease and AGD, it has been shown that filamentous tau appears to spread in a stereotypic manner as the disease progresses. We previously demonstrated that the injection of brain extracts from human mutant P301S tau-expressing transgenic mice into the brains of mice transgenic for wild-type human tau (line ALZ17) resulted in the assembly of wild-type human tau into filaments and the spreading of tau inclusions from the injection sites to anatomically connected brain regions. Here we injected brain extracts from humans who had died with various tauopathies into the hippocampus and cerebral cortex of ALZ17 mice. Argyrophilic tau inclusions formed in all cases and following the injection of the corresponding brain extracts, we recapitulated the hallmark lesions of AGD, PSP and CBD. Similar inclusions also formed after intracerebral injection of brain homogenates from human tauopathies into nontransgenic mice. Moreover, the induced formation of tau aggregates could be propagated between mouse brains. These findings suggest that once tau aggregates have formed in discrete brain areas, they become self-propagating and spread in a prion-like manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33(7):317–325. - PubMed
-
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. - PubMed
-
- Goedert M, Spillantini MG, Cairns NJ, Crowther RA. Tau proteins of Alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron. 1992;8(1):159–168. - PubMed
-
- Noda K, et al. Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): The Hisayama Study. Neuropathology. 2006;26(6):508–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

