Primary cilia: the chemical antenna regulating human adipose-derived stem cell osteogenesis
- PMID: 23690943
- PMCID: PMC3656889
- DOI: 10.1371/journal.pone.0062554
Primary cilia: the chemical antenna regulating human adipose-derived stem cell osteogenesis
Abstract
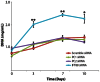
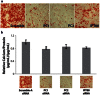
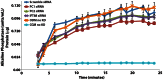
Adipose-derived stem cells (ASC) are multipotent stem cells that show great potential as a cell source for osteogenic tissue replacements and it is critical to understand the underlying mechanisms of lineage specification. Here we explore the role of primary cilia in human ASC (hASC) differentiation. This study focuses on the chemosensitivity of the primary cilium and the action of its associated proteins: polycystin-1 (PC1), polycystin-2 (PC2) and intraflagellar transport protein-88 (IFT88), in hASC osteogenesis. To elucidate cilia-mediated mechanisms of hASC differentiation, siRNA knockdown of PC1, PC2 and IFT88 was performed to disrupt cilia-associated protein function. Immunostaining of the primary cilium structure indicated phenotypic-dependent changes in cilia morphology. hASC cultured in osteogenic differentiation media yielded cilia of a more elongated conformation than those cultured in expansion media, indicating cilia-sensitivity to the chemical environment and a relationship between the cilium structure and phenotypic determination. Abrogation of PC1, PC2 and IFT88 effected changes in both hASC proliferation and differentiation activity, as measured through proliferative activity, expression of osteogenic gene markers, calcium accretion and endogenous alkaline phosphatase activity. Results indicated that IFT88 may be an early mediator of the hASC differentiation process with its knockdown increasing hASC proliferation and decreasing Runx2, alkaline phosphatase and BMP-2 mRNA expression. PC1 and PC2 knockdown affected later osteogenic gene and end-product expression. PC1 knockdown resulted in downregulation of alkaline phosphatase and osteocalcin gene expression, diminished calcium accretion and reduced alkaline phosphatase enzymatic activity. Taken together our results indicate that the structure of the primary cilium is intimately associated with the process of hASC osteogenic differentiation and that its associated proteins are critical players in this process. Elucidating the dynamic role of the primary cilium and its associated proteins will help advance the application of hASC in generating autologous tissue engineered therapies in critical defect bone injuries.
Conflict of interest statement
Figures





References
-
- Singla V (2006) The Primary Cilium as the Cell's Antenna: Signaling at a Sensory Organelle. Science 313: 629–633 doi:10.1126/science.1124534. - DOI - PubMed
-
- Pazour GJ, Witman GB (2003) The vertebrate primary cilium is a sensory organelle. Current Opinion in Cell Biology 15: 105–110 doi:10.1016/S0955-0674(02)00012-1. - DOI - PubMed
-
- Santos N, Reiter JF (2008) Building it up and taking it down: The regulation of vertebrate ciliogenesis. Dev Dyn 237: 1972–1981 doi:10.1002/dvdy.21540. - DOI - PMC - PubMed
-
- Eggenschwiler JT, Anderson KV (2007) Cilia and Developmental Signaling. Annu Rev Cell Dev Biol 23: 345–373 doi:10.1146/annurev.cellbio.23.090506.123249. - DOI - PMC - PubMed
-
- Abou Alaiwi WA, Lo ST, Nauli SM (2009) Primary Cilia: Highly Sophisticated Biological Sensors. Sensors 9: 7003–7020 doi:10.3390/s90907003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

