Simvastatin inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK and JAK2/STAT3 pathway
- PMID: 23690956
- PMCID: PMC3656850
- DOI: 10.1371/journal.pone.0062823
Simvastatin inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK and JAK2/STAT3 pathway
Abstract
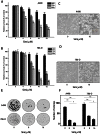
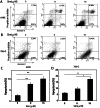
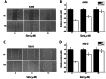
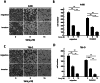
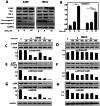
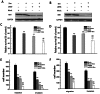
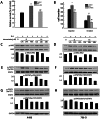
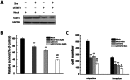
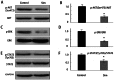
Renal cell carcinoma (RCC) is the most lethal type of genitourinary cancer due to its occult onset and resistance to chemotherapy and radiation. Recently, accumulating evidence has suggested stains, inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase, were associated with the risk reduction of cancer. In the present study, we aimed to investigate the potential effects of simvastatin on RCC cells and the underlying mechanisms by which simvastatin exerted its actions. With cell viability, colony formation, and flow cytometric apoptosis assays, we found that simvastatin potently suppressed cell growth of A498 and 786-O cells in a time- and dose- dependent manner. Consistently, the xenograft model performed in nude mice exhibited reduced tumor growth with simvastatin treatment. In addition, the inhibitory effects of simvastatin on migration and invasion were also observed in vitro. Mechanically, we presented that simvastatin could suppress the proliferation and motility of RCC cells via inhibiting the phosphorylation of AKT, mTOR, and ERK in a time- and dose- dependent manner. Further investigation of the underlying mechanism revealed simvastatin could exert the anti-tumor effects by suppressing IL-6-induced phosphorylation of JAK2 and STAT3. In conclusion, these findings suggested that simvastatin-induced apoptosis and its anti-metastasis activity in RCC cells were accompanied by inhibition of AKT/mTOR, ERK, and JAK2/STAT3 pathways, which imply that simvastatin may be a potential therapeutic agent for the treatment of RCC patients.
Conflict of interest statement
Figures

References
-
- Bex A, Jonasch E, Kirkali Z, Mejean A, Mulders P, et al. (2010) Integrating surgery with targeted therapies for renal cell carcinoma: current evidence and ongoing trials. Eur Urol 58: 819–828. - PubMed
-
- Basso M, Cassano A, Barone C (2010) A survey of therapy for advanced renal cell carcinoma. Urol Oncol 28: 121–133. - PubMed
-
- Lam JS, Leppert JT, Belldegrun AS, Figlin RA (2005) Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol 23: 202–212. - PubMed
-
- Linehan WM, Zbar B (2004) Focus on kidney cancer. Cancer Cell 6: 223–228. - PubMed
-
- Motzer RJ, Russo P (2000) Systemic therapy for renal cell carcinoma. J Urol 163: 408–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

