Assessment and in vivo scoring of murine experimental autoimmune uveoretinitis using optical coherence tomography
- PMID: 23690973
- PMCID: PMC3653962
- DOI: 10.1371/journal.pone.0063002
Assessment and in vivo scoring of murine experimental autoimmune uveoretinitis using optical coherence tomography
Abstract
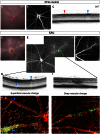
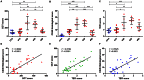
Despite advances in clinical imaging and grading our understanding of retinal immune responses and their morphological correlates in experimental autoimmune uveoretinitis (EAU), has been hindered by the requirement for post-mortem histology. To date, monitoring changes occurring during EAU disease progression and evaluating the effect of therapeutic intervention in real time has not been possible. We wanted to establish whether optical coherence tomography (OCT) could detect intraretinal changes during inflammation and to determine its utility as a tool for accurate scoring of EAU. EAU was induced in C57BL/6J mice and animals evaluated after 15, 26, 36 and 60 days. At each time-point, contemporaneous Spectralis-OCT scanning, topical endoscopic fundal imaging (TEFI), fundus fluorescein angiography (FFA) and CD45-immunolabelled histology were performed. OCT features were further characterised on retinal flat-mounts using immunohistochemistry and 3D reconstruction. Optic disc swelling and vitreous opacities detected by OCT corresponded to CD45+ cell infiltration on histology. Vasculitis identified by FFA and OCT matched perivascular myeloid and T-cell infiltrates and could be differentiated from unaffected vessels. Evolution of these changes could be followed over time in the same eye. Retinal folds were visible and found to encapsulate mixed populations of activated myeloid cells, T-cells and microglia. Using these features, an OCT-based EAU scoring system was developed, with significant correlation to validated histological (Pearson r(2) = 0.6392, P<0.0001, n = 31 eyes) and TEFI based scoring systems (r(2) = 0.6784, P<0.0001). OCT distinguishes the fundamental features of murine EAU in vivo, permits dynamic assessment of intraretinal changes and can be used to score disease severity. As a result, it allows tissue synchronisation with subsequent cellular and functional assessment and greater efficiency of animal usage. By relating OCT signals with immunohistochemistry in EAU, our findings offer the opportunity to inform the interpretation of OCT changes in human uveitis.
Conflict of interest statement
Figures



References
-
- Schewitz-Bowers LP, Lee RW, Dick AD (2010) Immune mechanisms of intraocular inflammation. Ex Rev Op 5: 43–58.
-
- Calder CJ, Nicholson LB, Dick AD (2005) A selective role for the TNF p55 receptor in autocrine signaling following IFN-gamma stimulation in experimental autoimmune uveoretinitis. J Immunol 175: 6286–6293. - PubMed
-
- Raveney BJE, Copland DA, Dick AD, Nicholson LB (2009) TNFR1-dependent regulation of myeloid cell function in experimental autoimmune uveoretinitis. J Immunol 183: 2321–2329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

