Septins are important for cell polarity, septation and asexual spore formation in Neurospora crassa and show different patterns of localisation at germ tube tips
- PMID: 23691103
- PMCID: PMC3653863
- DOI: 10.1371/journal.pone.0063843
Septins are important for cell polarity, septation and asexual spore formation in Neurospora crassa and show different patterns of localisation at germ tube tips
Abstract
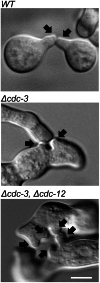
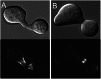
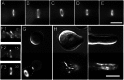
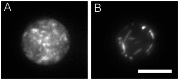
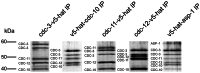
Septins are GTP-binding cytoskeletal proteins that contribute to cell polarity, vesicle trafficking, cytokinesis and cell morphogenesis. Here we have characterised the six septins encoded by the genome of the model filamentous fungus Neurospora crassa. Analysis of septin null mutants demonstrated that septins limit the sites of emergence of germ tubes and are important for septation and conidiation in N. crassa. Septins constituted a range of different higher-order structures in N. crassa - rings, loops, fibres, bands, and caps - which can co-exist within the same cell. They showed different patterns of localisation at germ tube tips, with GFP-CDC-10 and CDC-11-GFP forming a subapical collar with lower signal intensity at the tip apex, CDC-3-GFP and CDC-12-GFP organized as a cap at the tip apex and GFP-ASP-1 forming an extended subapical collar. Purification of the septin complex and mass spectrometry of isolated proteins revealed that the septin complex consists predominantly of CDC-3, CDC-10, CDC-11 and CDC-12. Immunoprecipitation of the putative septin ASP-1 revealed that this protein interacts with the core septin complex.
Conflict of interest statement
Figures







References
-
- Hartwell LH (1971) Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res 69: 265–276. - PubMed
-
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, et al. (2007) Structural insight into filament formation by mammalian septins. Nature 449: 311–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

