Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance
- PMID: 23691241
- PMCID: PMC3653867
- DOI: 10.1371/journal.pone.0064521
Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance
Abstract
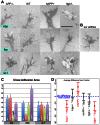
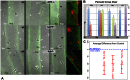
Amyloid precursor protein (APP), a transmembrane glycoprotein, is well known for its involvement in the pathogenesis of Alzheimer disease of the aging brain, but its normal function is unclear. APP is a prominent component of the adult as well as the developing brain. It is enriched in axonal growth cones (GCs) and has been implicated in cell adhesion and motility. We tested the hypothesis that APP is an extracellular matrix adhesion molecule in experiments that isolated the function of APP from that of well-established adhesion molecules. To this end we plated wild-type, APP-, or β1-integrin (Itgb1)- misexpressing mouse hippocampal neurons on matrices of either laminin, recombinant L1, or synthetic peptides binding specifically to Itgb1 s or APP. We measured GC adhesion, initial axonal outgrowth, and substrate preference on alternating matrix stripes and made the following observations: Substrates of APP-binding peptide alone sustain neurite outgrowth; APP dosage controls GC adhesion to laminin and APP-binding peptide as well as axonal outgrowth in Itgb1- independent manner; and APP directs GCs in contact guidance assays. It follows that APP is an independently operating cell adhesion molecule that affects the GC's phenotype on APP-binding matrices including laminin, and that it is likely to affect axon pathfinding in vivo.
Conflict of interest statement
Figures

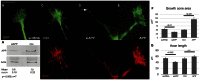
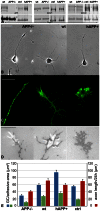
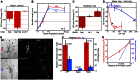
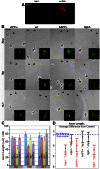


References
-
- De Strooper B, Annaert W (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci 113 (Pt 11): 1857–1870. - PubMed
-
- Beher D, Hesse L, Masters CL, Multhaup G (1996) Regulation of amyloid protein precursor (APP) binding to collagen and mapping of the binding sites on APP and collagen type I. J Biol Chem. 271: 1613–1620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

