The Gut's Little Brain in Control of Intestinal Immunity
- PMID: 23691339
- PMCID: PMC3649343
- DOI: 10.1155/2013/630159
The Gut's Little Brain in Control of Intestinal Immunity
Abstract
The gut immune system shares many mediators and receptors with the autonomic nervous system. Good examples thereof are the parasympathetic (vagal) and sympathetic neurotransmitters, for which many immune cell types in a gut context express receptors or enzymes required for their synthesis. For some of these the relevance for immune regulation has been recently defined. Earlier and more recent studies in neuroscience and immunology have indicated the anatomical and cellular basis for bidirectional interactions between the nervous and immune systems. Sympathetic immune modulation is well described earlier, and in the last decade the parasympathetic vagal nerve has been put forward as an integral part of an immune regulation network via its release of Ach, a system coined "the cholinergic anti-inflammatory reflex." A prototypical example is the inflammatory reflex, comprised of an afferent arm that senses inflammation and an efferent arm: the cholinergic anti-inflammatory pathway, that inhibits innate immune responses. In this paper, the current understanding of how innate mucosal immunity can be influenced by the neuronal system is summarized, and cell types and receptors involved in this interaction will be highlighted. Focus will be given on the direct neuronal regulatory mechanisms, as well as current advances regarding the role of microbes in modulating communication in the gut-brain axis.
Figures


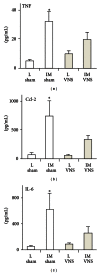
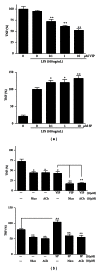
References
-
- Dhawan S, Cailotto C, Harthoorn LF, de Jonge WJ. Cholinergic signalling in gut immunity. Life Sciences. 2012;91(21-22):1038–1042. - PubMed
-
- Fujii T, Takada-Takatori Y, Kawashima K. Regulatory mechanisms of acetylcholine synthesis and release by T cells. Life Sciences. 2012;91(21-22):981–985. - PubMed
-
- Elenkov IJ, Chrousos GP. Stress system—organization, physiology and immunoregulation. NeuroImmunoModulation. 2007;13(5-6):257–267. - PubMed
-
- The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacological Reviews. 2000;52:595–638. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

