A pharmacist-led system-change smoking cessation intervention for smokers admitted to Australian public hospitals (GIVE UP FOR GOOD): study protocol for a randomised controlled trial
- PMID: 23693155
- PMCID: PMC3664596
- DOI: 10.1186/1745-6215-14-148
A pharmacist-led system-change smoking cessation intervention for smokers admitted to Australian public hospitals (GIVE UP FOR GOOD): study protocol for a randomised controlled trial
Abstract
Background: Intensive smoking cessation interventions initiated during hospitalisation are effective, but currently not widely available. Strategies are needed to integrate smoking cessation treatment into routine inpatient care. Pharmacist-led interventions for smoking cessation are feasible and efficacious in both ambulatory and community pharmacy settings. However, there is a lack of evidence from large scale studies of the effectiveness of pharmacist guided programs initiated during patient hospitalisation in achieving long-term abstinence. This study aims to evaluate the effectiveness of a pharmacist-led system change intervention initiated during hospitalisation in Australian public hospitals.
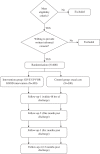
Methods/design: A multi-centre, randomised controlled trial will be conducted with 12 months follow-up. Smokers, 18 years or older, will be recruited from the wards of three Victorian public hospitals. Participants will be randomly assigned to a usual care or intervention group using a computer generated randomisation list. The intervention group will receive at least three smoking cessation support sessions by a trained pharmacist: the first during the hospital stay, the second on or immediately after discharge and the third within one month post-discharge. All smoking cessation medications will be provided free of charge during the hospital stay and for at least one week after discharge. Participants randomised to usual care will receive the current care routinely provided by the hospital. All measurements at baseline, discharge, one, six and 12 months will be performed by a blinded Research Assistant. The primary outcome measures are carbon monoxide validated 7-day point prevalence abstinence at six and 12 months.
Discussion: This is the first large scale study to develop and test a pharmacist-led system change intervention program initiated during patient hospitalisation. If successful, the program could be considered for wider implementation across other hospitals.
Trial registration: ACTRN12612000368831.
Figures
References
-
- World Health Organisation. WHO Report on the Global Tobacco Epidemic: Warning about the Dangers of Tobacco. Geneva: World Health Organisation; 2011.
-
- Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez AD. The Burden of Disease and Injury in Australia 2003. Canberra: Australian Institute of Health and Welfare; 2007.
-
- Collins DJ, Lapsley HM. The Costs of Tobacco, Alcohol and Illicit Drug Abuse to Australian Society in 2004–05. Canberra: Department of Health and Ageing; 2008.
-
- Australian Institute of Health and Welfare. Australia’s Health 2012. The Thirteenth Biennial Health Report of the Australian Institute of Health and Welfare. Canberra: Australian Institute of Health and Welfare; 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical