Structure and dynamics of an imidazoline nitroxide side chain with strongly hindered internal motion in proteins
- PMID: 23694751
- PMCID: PMC3758229
- DOI: 10.1016/j.jmr.2013.04.013
Structure and dynamics of an imidazoline nitroxide side chain with strongly hindered internal motion in proteins
Abstract
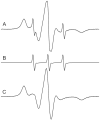
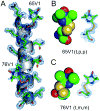
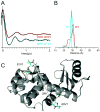
A disulfide-linked imidazoline nitroxide side chain (V1) has a similar and highly constrained internal motion at diverse topological sites in a protein, unlike that for the disulfide-linked pyrroline nitroxide side chain (R1) widely used in site directed spin labeling EPR. Crystal structures of V1 at two positions in a helix of T4 Lysozyme and quantum mechanical calculations suggest the source of the constraints as intra-side chain interactions of the disulfide sulfur atoms with both the protein backbone and the 3-nitrogen in the imidazoline ring. These interactions apparently limit the conformation of the side chain to one of only three possible rotamers, two of which are observed in the crystal structure. An inter-spin distance measurement in frozen solution using double electron-electron resonance (DEER) gives a value essentially identical to that determined from the crystal structure of the protein containing two copies of V1, indicating that lattice forces do not dictate the rotamers observed. Collectively, the results suggest the possibility of predetermining a unique rotamer of V1 in helical structures. In general, the reduced rotameric space of V1 compared to R1 should simplify interpretation of inter-spin distance information in terms of protein structure, while the highly constrained internal motion is expected to extend the dynamic range for characterizing large amplitude nanosecond backbone fluctuations.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Crystal structures of spin labeled T4 lysozyme mutants: implications for the interpretation of EPR spectra in terms of structure.Biochemistry. 2000 Jul 25;39(29):8396-405. doi: 10.1021/bi000604f. Biochemistry. 2000. PMID: 10913245
-
Structural determinants of nitroxide motion in spin-labeled proteins: solvent-exposed sites in helix B of T4 lysozyme.Protein Sci. 2008 Feb;17(2):228-39. doi: 10.1110/ps.073174008. Epub 2007 Dec 20. Protein Sci. 2008. PMID: 18096642 Free PMC article.
-
Structural origin of weakly ordered nitroxide motion in spin-labeled proteins.Protein Sci. 2009 May;18(5):893-908. doi: 10.1002/pro.96. Protein Sci. 2009. PMID: 19384990 Free PMC article.
-
Nitroxide spin labels and EPR spectroscopy: A powerful association for protein dynamics studies.Biochim Biophys Acta Proteins Proteom. 2021 Jul;1869(7):140653. doi: 10.1016/j.bbapap.2021.140653. Epub 2021 Mar 20. Biochim Biophys Acta Proteins Proteom. 2021. PMID: 33757896 Review.
-
Identifying conformational changes with site-directed spin labeling.Nat Struct Biol. 2000 Sep;7(9):735-9. doi: 10.1038/78956. Nat Struct Biol. 2000. PMID: 10966640 Review.
Cited by
-
DEER Analysis of GPCR Conformational Heterogeneity.Biomolecules. 2021 May 22;11(6):778. doi: 10.3390/biom11060778. Biomolecules. 2021. PMID: 34067265 Free PMC article. Review.
-
Full engagement of liganded maltose-binding protein stabilizes a semi-open ATP-binding cassette dimer in the maltose transporter.Mol Microbiol. 2015 Dec;98(5):878-94. doi: 10.1111/mmi.13165. Epub 2015 Sep 10. Mol Microbiol. 2015. PMID: 26268698 Free PMC article.
-
Structural basis of arrestin-3 activation and signaling.Nat Commun. 2017 Nov 10;8(1):1427. doi: 10.1038/s41467-017-01218-8. Nat Commun. 2017. PMID: 29127291 Free PMC article.
-
Spectroscopically Orthogonal Labelling to Disentangle Site-Specific Nitroxide Label Distributions.Appl Magn Reson. 2024;55(1-3):187-205. doi: 10.1007/s00723-023-01611-1. Epub 2023 Sep 24. Appl Magn Reson. 2024. PMID: 38357007 Free PMC article.
-
A Comparison of Cysteine-Conjugated Nitroxide Spin Labels for Pulse Dipolar EPR Spectroscopy.Molecules. 2021 Dec 13;26(24):7534. doi: 10.3390/molecules26247534. Molecules. 2021. PMID: 34946616 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

