Simultaneous structure-activity studies and arming of natural products by C-H amination reveal cellular targets of eupalmerin acetate
- PMID: 23695633
- PMCID: PMC4041531
- DOI: 10.1038/nchem.1653
Simultaneous structure-activity studies and arming of natural products by C-H amination reveal cellular targets of eupalmerin acetate
Erratum in
- Nat Chem. 2013 Jul;5(7):634
Abstract
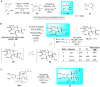
Natural products have a venerable history of, and enduring potential for the discovery of useful biological activity. To fully exploit this, the development of chemical methodology that can functionalize unique sites within these complex structures is highly desirable. Here, we describe the use of rhodium(II)-catalysed C-H amination reactions developed by Du Bois to carry out simultaneous structure-activity relationship studies and arming (alkynylation) of natural products at 'unfunctionalized' positions. Allylic and benzylic C-H bonds in the natural products undergo amination while olefins undergo aziridination, and tertiary amine-containing natural products are converted to amidines by a C-H amination-oxidation sequence or to hydrazine sulfamate zwitterions by an unusual N-amination. The alkynylated derivatives are ready for conversion into cellular probes that can be used for mechanism-of-action studies. Chemo- and site-selectivity was studied with a diverse library of natural products. For one of these-the marine-derived anticancer diterpene, eupalmerin acetate-quantitative proteome profiling led to the identification of several protein targets in HL-60 cells, suggesting a polypharmacological mode of action.
Figures
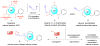


References
-
- Li JWH, Vederas JC. Drug discovery and natural products: End of an era or an endless frontier? Science. 2009;325:161–165. - PubMed
-
-
For a recent special issue on this topic, see: Schreiber SL. Organic synthesis toward small-molecule probes and drugs, special feature. Proc Nat Acad Sci. 2011;108:6699–6822.
-
-
- Koehn FE. High impact technologies for natural products screening. Prog Drug Res. 2008;65:177–210. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/162163319
- PubChem-Substance/162163320
- PubChem-Substance/162163321
- PubChem-Substance/162163322
- PubChem-Substance/162163323
- PubChem-Substance/162163324
- PubChem-Substance/162163325
- PubChem-Substance/162163326
- PubChem-Substance/162163327
- PubChem-Substance/162163328
- PubChem-Substance/162163329
- PubChem-Substance/162163330
- PubChem-Substance/162163331
- PubChem-Substance/162163332
- PubChem-Substance/162163333
- PubChem-Substance/162163334
- PubChem-Substance/162163335
- PubChem-Substance/162163336
- PubChem-Substance/162163337
- PubChem-Substance/162163338
- PubChem-Substance/162163339
- PubChem-Substance/162163340
- PubChem-Substance/162163341
- PubChem-Substance/162163342
- PubChem-Substance/162163343
- PubChem-Substance/162163344
- PubChem-Substance/162163345
- PubChem-Substance/162163346
- PubChem-Substance/162163347
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

