Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation
- PMID: 23695680
- PMCID: PMC3842019
- DOI: 10.1038/ncomms2877
Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation
Abstract
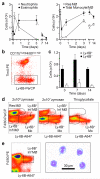
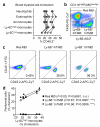
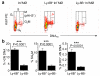
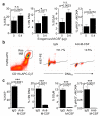
The general paradigm is that monocytes are recruited to sites of inflammation and terminally differentiate into macrophages. There has been no demonstration of proliferation of peripherally-derived inflammatory macrophages under physiological conditions. Here we show that proliferation of both bone marrow-derived inflammatory and tissue-resident macrophage lineage branches is a key feature of the inflammatory process with major implications for the mechanisms underlying recovery from inflammation. Both macrophage lineage branches are dependent on M-CSF during inflammation, and thus the potential for therapeutic interventions is marked. Furthermore, these observations are independent of Th2 immunity. These studies indicate that the proliferation of distinct macrophage populations provides a general mechanism for macrophage expansion at key stages during inflammation, and separate control mechanisms are implicated.
Figures

References
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
-
- Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. - PubMed
-
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. - PubMed
-
- Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

