Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials
- PMID: 23695688
- PMCID: PMC3674274
- DOI: 10.1038/ncomms2932
Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials
Abstract
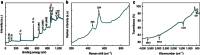
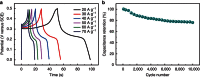
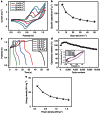
Among numerous active electrode materials, nickel hydroxide is a promising electrode in electrochemical capacitors. Nickel hydroxide research has thus far focused on the crystalline rather than the amorphous phase, despite the impressive electrochemical properties of the latter, which includes an improved electrochemical efficiency due to disorder. Here we demonstrate high-performance electrochemical supercapacitors prepared from amorphous nickel hydroxide nanospheres synthesized via simple, green electrochemistry. The amorphous nickel hydroxide electrode exhibits high capacitance (2,188 F g(-1)), and the asymmetric pseudocapacitors of the amorphous nickel hydroxide exhibit high capacitance (153 F g(-1)), high energy density (35.7 W h kg(-1) at a power density of 490 W kg(-1)) and super-long cycle life (97% and 81% charge retentions after 5,000 and 10,000 cycles, respectively). The integrated electrochemical performance of the amorphous nickel hydroxide is commensurate with crystalline materials in supercapacitors. These findings promote the application of amorphous nanostructures as advanced electrochemical pseudocapacitor materials.
Figures


References
-
- Winter M. & Brodd R. J. What are batteries, fuel cells, and supercapacitors. Chem. Rev. 104, 4245–4269 (2004). - PubMed
-
- Liu D. et al.. Hydrous manganese dioxide nanowall arrays growth and their Li+ ions intercalation electrochemical properties. Chem. Mater. 20, 1376–1380 (2008).
-
- Miller J. R. & Simon P. Electrochemical capacitors for energy management. Science 321, 651–652 (2008). - PubMed
-
- Wang H. L. Casalongue H. S. Liang Y. Y. & Dai H. J. Ni(OH)2 Nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132, 7472–7477 (2010). - PubMed
-
- Ramesh T. N. Jayashree R. S. Kamath P. V. Rodrigues S. & Shukla A. K. Effect of lightweight supports on specific discharge capacity of nickel hydroxide. J. Power Sources 104, 295–298 (2002).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

