An automated climbing apparatus to measure chemotherapy-induced neurotoxicity in Drosophila melanogaster
- PMID: 23695893
- PMCID: PMC4049852
- DOI: 10.4161/fly.24789
An automated climbing apparatus to measure chemotherapy-induced neurotoxicity in Drosophila melanogaster
Abstract
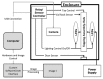
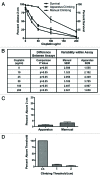
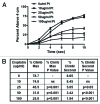
We have developed a novel model system in Drosophila melanogaster to study chemotherapy-induced neurotoxicity in adult flies. Neurological deficits were measured using a manual geotactic climbing assay. The manual assay is commonly used; however, it is laborious, time-consuming, subject to human error and limited to observing one sample at a time. We have designed and built a new automated fly-counting apparatus that uses a "video capture-particle counting technology" to automatically measure 10 samples at a time, with 20 flies per sample. Climbing behavior was assessed manually, as in our previous studies, and with the automated apparatus within the same experiment yielding statistically similar results. Both climbing endpoints as well as the climbing rate can be measured in the apparatus, giving the assay more versatility than the manual assay. Automation of our climbing assay reduces variability, increases productivity and enables high throughput drug screens for neurotoxicity.
Keywords: Drosophila; apparatus; automated; chemotherapy; cisplatin; climbing; geotactic; neurotoxicity.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
