Decreasing incidence of symptomatic Epstein-Barr virus disease and posttransplant lymphoproliferative disorder in pediatric liver transplant recipients: report of the studies of pediatric liver transplantation experience
- PMID: 23696264
- PMCID: PMC5001558
- DOI: 10.1002/lt.23659
Decreasing incidence of symptomatic Epstein-Barr virus disease and posttransplant lymphoproliferative disorder in pediatric liver transplant recipients: report of the studies of pediatric liver transplantation experience
Abstract
Posttransplant lymphoproliferative disorder (PTLD) causes significant morbidity and mortality in pediatric recipients of liver transplantation (LT).
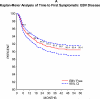
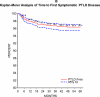
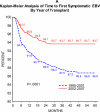
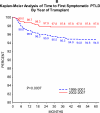
Objective: Describe the incidence of PTLD and symptomatic Epstein-Barr virus (SEBV) disease in a large multicenter cohort of children who underwent LT with a focus on the risk factors and changes in incidence over time. SEBV and PTLD were prospectively determined in 2283 subjects who had undergone LT for the first time with at least 1 year of follow-up in the Studies of Pediatric Liver Transplantation database. SEBV was defined with specific criteria, and PTLD required tissue confirmation. The incidence of SEBV and PTLD was determined with a Kaplan-Meier analysis. Univariate and multivariate modeling of risk factors was performed with standard methods. SEBV occurred in 199 patients; 174 (87.4%) were EBV-negative at LT. Seventy-five patients developed PTLD, and 64 (85.3%) of these patients were EBV-negative at LT. Among the 2048 patients with at least 2 years of follow-up, 8.3% developed SEBV by the second year after LT, and 2.8% developed PTLD. There were lower rates of SEBV (5.9% versus 11.3%, P < 0.001) and PTLD (1.7% versus 4.2%, P = 0.001) in 2002-2007 versus 1995-2001. In 2002-2007, tacrolimus and cyclosporine trough blood levels in the first year after LT were significantly lower, and fewer children were receiving steroids. Biliary atresia, and recipient EBV status were correlated. In a multivariate analysis, era of LT, recipient EBV status, and frequent rejection episodes were associated with SEBV and PTLD. The incidence of SEBV and PTLD is decreasing in pediatric LT recipients concomitantly with a reduction in immunosuppression. Younger recipients and those with multiple rejections remain at higher risk for SEBV and PTLD.
© 2013 American Association for the Study of Liver Diseases.
Figures
References
-
- Cox KL, Lawrence-Miyasaki LS, Garcia-Kennedy R, Lennette ET, Martinez OM, Krams SM, et al. An increased incidence of Epstein-Barr virus infection and lymphoproliferative disorder in young children on FK506 after liver transplantation. Transplantation. 1995;59:524–9. - PubMed
-
- Guthery SL, Heubi JE, Bucuvalas JC, Gross TG, Ryckman FC, Alonso MH, et al. Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation. 2003;75:987–93. - PubMed
-
- Sokal EM, Antunes H, Beguin C, Bodeus M, Wallemacq P, de Ville de Goyet J, et al. Early signs and risk factors for the increased incidence of Epstein-Barr virus-related posttransplant lymphoproliferative diseases in pediatric liver transplant recipients treated with tacrolimus. Transplantation. 1997;64:1438–42. - PubMed
-
- Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, et al. Posttransplant lymphoproliferative disease in pediatric liver transplantation. Interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62:370–5. - PubMed
-
- Cacciarelli TV, Reyes J, Jaffe R, Mazariegos GV, Jain A, Fung JJ, et al. Primary tacrolimus (FK506) therapy and the long-term risk of post-transplant lymphoproliferative disease in pediatric liver transplant recipients. Pediatr Transplant. 2001;5:359–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
