Stem cells in the Drosophila digestive system
- PMID: 23696352
- PMCID: PMC7571253
- DOI: 10.1007/978-94-007-6621-1_5
Stem cells in the Drosophila digestive system
Abstract
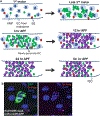
Adult stem cells maintain tissue homeostasis by continuously replenishing damaged, aged and dead cells in any organism. Five types of region and organ-specific multipotent adult stem cells have been identified in the Drosophila digestive system: intestinal stem cells (ISCs) in the posterior midgut; hindgut intestinal stem cells (HISCs) at the midgut/hindgut junction; renal and nephric stem cells (RNSCs) in the Malpighian Tubules; type I gastric stem cells (GaSCs) at foregut/midgut junction; and type II gastric stem cells (GSSCs) at the middle of the midgut. Despite the fact that each type of stem cell is unique to a particular organ, they share common molecular markers and some regulatory signaling pathways. Due to the simpler tissue structure, ease of performing genetic analysis, and availability of abundant mutants, Drosophila serves as an elegant and powerful model system to study complex stem cell biology. The recent discoveries, particularly in the Drosophila ISC system, have greatly advanced our understanding of stem cell self-renewal, differentiation, and the role of stem cells play in tissue homeostasis/regeneration and adaptive tissue growth.
Figures




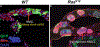
References
-
- Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100(1):157–168 - PubMed
-
- Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol 55:593–608 - PubMed
-
- Saric A, Kalafatic M, Rusak G, Kovacevic G et al. (2007) Postembryonic development of Drosophila melanogaster Meigen, 1830 under the influence of quercetin. Entomol News 118(3):235–240
-
- Slama L, Farkas R (2005) Heartbeat patterns during the postembryonic development of Drosophila melanogaster. J Insect Physiol 51(5):489–503 - PubMed
-
- Yamashita Y (2009) Asymmetric stem cell division and pathology: insights from Drosophila stem cell systems. J Pathol 217(2):181–185 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

