Vitamin D nuclear receptor deficiency promotes cholestatic liver injury by disruption of biliary epithelial cell junctions in mice
- PMID: 23696511
- PMCID: PMC4286017
- DOI: 10.1002/hep.26453
Vitamin D nuclear receptor deficiency promotes cholestatic liver injury by disruption of biliary epithelial cell junctions in mice
Abstract
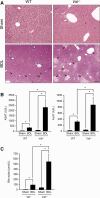
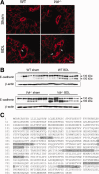
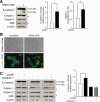
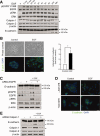
Alterations in apical junctional complexes (AJCs) have been reported in genetic or acquired biliary diseases. The vitamin D nuclear receptor (VDR), predominantly expressed in biliary epithelial cells in the liver, has been shown to regulate AJCs. The aim of our study was thus to investigate the role of VDR in the maintenance of bile duct integrity in mice challenged with biliary-type liver injury. Vdr(-/-) mice subjected to bile duct ligation (BDL) displayed increased liver damage compared to wildtype BDL mice. Adaptation to cholestasis, ascertained by expression of genes involved in bile acid metabolism and tissue repair, was limited in Vdr(-/-) BDL mice. Furthermore, evaluation of Vdr(-/-) BDL mouse liver tissue sections indicated altered E-cadherin staining associated with increased bile duct rupture. Total liver protein analysis revealed that a truncated form of E-cadherin was present in higher amounts in Vdr(-/-) mice subjected to BDL compared to wildtype BDL mice. Truncated E-cadherin was also associated with loss of cell adhesion in biliary epithelial cells silenced for VDR. In these cells, E-cadherin cleavage occurred together with calpain 1 activation and was prevented by the silencing of calpain 1. Furthermore, VDR deficiency led to the activation of the epidermal growth factor receptor (EGFR) pathway, while EGFR activation by EGF induced both calpain 1 activation and E-cadherin cleavage in these cells. Finally, truncation of E-cadherin was blunted when EGFR signaling was inhibited in VDR-silenced cells.
Conclusion: Biliary-type liver injury is exacerbated in Vdr(-/-) mice by limited adaptive response and increased bile duct rupture. These results indicate that loss of VDR restricts the adaptation to cholestasis and diminishes bile duct integrity in the setting of biliary-type liver injury.
Copyright © 2013 The Authors. HEPATOLOGY published by Wiley on behalf of the American Association for the Study of Liver Diseases.
Figures



Similar articles
-
Plectin controls biliary tree architecture and stability in cholestasis.J Hepatol. 2018 May;68(5):1006-1017. doi: 10.1016/j.jhep.2017.12.011. Epub 2017 Dec 20. J Hepatol. 2018. PMID: 29273475
-
Yes-associated protein regulates the hepatoprotective effect of vitamin D receptor activation through promoting adaptive bile duct remodeling in cholestatic mice.J Pathol. 2021 Sep;255(1):95-106. doi: 10.1002/path.5750. Epub 2021 Jul 16. J Pathol. 2021. PMID: 34156701
-
Impairment of bilirubin clearance and intestinal interleukin-6 expression in bile duct-ligated vitamin D receptor null mice.PLoS One. 2012;7(12):e51664. doi: 10.1371/journal.pone.0051664. Epub 2012 Dec 11. PLoS One. 2012. PMID: 23240054 Free PMC article.
-
Animal models of biliary tract injury.Curr Opin Gastroenterol. 2012 May;28(3):239-43. doi: 10.1097/MOG.0b013e32835264d9. Curr Opin Gastroenterol. 2012. PMID: 22450892 Review.
-
Animal models of biliary injury and altered bile acid metabolism.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1254-1261. doi: 10.1016/j.bbadis.2017.06.027. Epub 2017 Jul 11. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28709963 Free PMC article. Review.
Cited by
-
Vitamin D treatment modulates immune activation in cystic fibrosis.Clin Exp Immunol. 2017 Sep;189(3):359-371. doi: 10.1111/cei.12984. Epub 2017 May 24. Clin Exp Immunol. 2017. PMID: 28470739 Free PMC article. Clinical Trial.
-
Calcipotriol Inhibits NLRP3 Signal Through YAP1 Activation to Alleviate Cholestatic Liver Injury and Fibrosis.Front Pharmacol. 2020 Mar 31;11:200. doi: 10.3389/fphar.2020.00200. eCollection 2020. Front Pharmacol. 2020. PMID: 32296329 Free PMC article.
-
Pathophysiological Role and Therapeutic Implications of Vitamin D in Autoimmunity: Focus on Chronic Autoimmune Diseases.Nutrients. 2020 Mar 17;12(3):789. doi: 10.3390/nu12030789. Nutrients. 2020. PMID: 32192175 Free PMC article. Review.
-
Bile Acids and Biliary Fibrosis.Cells. 2023 Mar 2;12(5):792. doi: 10.3390/cells12050792. Cells. 2023. PMID: 36899928 Free PMC article. Review.
-
Exploration of the Combined Mechanism of Direct and Indirect Effects of Paeoniflorin in the Treatment of Cholestasis.Inflammation. 2025 Jan 27. doi: 10.1007/s10753-025-02245-0. Online ahead of print. Inflammation. 2025. PMID: 39869299
References
-
- Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–1390. - PubMed
-
- Grosse B, Cassio D, Yousef N, Bernardo C, Jacquemin E, Gonzales E. Claudin-1 involved in neonatal ichthyosis sclerosing cholangitis syndrome regulates hepatic paracellular permeability. Hepatology. 2012;55:1249–1259. - PubMed
-
- Sakisaka S, Kawaguchi T, Taniguchi E, Hanada S, Sasatomi K, Koga H, et al. Alterations in tight junctions differ between primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2001;33:1460–1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
