Breaking the carboxyl rule: lysine 96 facilitates reprotonation of the Schiff base in the photocycle of a retinal protein from Exiguobacterium sibiricum
- PMID: 23696649
- PMCID: PMC3774394
- DOI: 10.1074/jbc.M113.465138
Breaking the carboxyl rule: lysine 96 facilitates reprotonation of the Schiff base in the photocycle of a retinal protein from Exiguobacterium sibiricum
Abstract
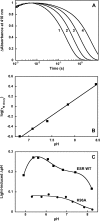
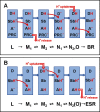
A lysine instead of the usual carboxyl group is in place of the internal proton donor to the retinal Schiff base in the light-driven proton pump of Exiguobacterium sibiricum (ESR). The involvement of this lysine in proton transfer is indicated by the finding that its substitution with alanine or other residues slows reprotonation of the Schiff base (decay of the M intermediate) by more than 2 orders of magnitude. In these mutants, the rate constant of the M decay linearly decreases with a decrease in proton concentration, as expected if reprotonation is limited by the uptake of a proton from the bulk. In wild type ESR, M decay is biphasic, and the rate constants are nearly pH-independent between pH 6 and 9. Proton uptake occurs after M formation but before M decay, which is especially evident in D2O and at high pH. Proton uptake is biphasic; the amplitude of the fast phase decreases with a pKa of 8.5 ± 0.3, which reflects the pKa of the donor during proton uptake. Similarly, the fraction of the faster component of M decay decreases and the slower one increases, with a pKa of 8.1 ± 0.2. The data therefore suggest that the reprotonation of the Schiff base in ESR is preceded by transient protonation of an initially unprotonated donor, which is probably the ε-amino group of Lys-96 or a water molecule in its vicinity, and it facilitates proton delivery from the bulk to the reaction center of the protein.
Keywords: Bioenergetics; Internal Proton Donor; Photobiology; Proton Pumps; Proton Transport; Retinal Proteins; Spectroscopy.
Figures





References
-
- Petrovskaya L. E., Lukashev E. P., Chupin V. V., Sychev S. V., Lyukmanova E. N., Kryukova E. A., Ziganshin R. H., Spirina E. V., Rivkina E. M., Khatypov R. A., Erokhina L. G., Gilichinsky D. A., Shuvalov V. A., Kirpichnikov M. P. (2010) Predicted bacteriorhodopsin from Exiguobacterium sibiricum is a functional proton pump. FEBS Lett. 584, 4193–4196 - PubMed
-
- Balashov S. P., Petrovskaya L. E., Lukashev E. P., Imasheva E. S., Dioumaev A. K., Wang J. M., Sychev S. V., Dolgikh D. A., Rubin A. B., Kirpichnikov M. P., Lanyi J. K. (2012) Aspartate-histidine interaction in the retinal Schiff base counterion of the light-driven proton pump of Exiguobacterium sibiricum. Biochemistry 51, 5748–5762 - PMC - PubMed
-
- Vishnivetskaya T., Kathariou S., McGrath J., Gilichinsky D., Tiedje J. M. (2000) Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments. Extremophiles 4, 165–173 - PubMed
-
- Rodrigues D. F., Goris J., Vishnivetskaya T., Gilichinsky D., Thomashow M. F., Tiedje J. M. (2006) Characterization of Exiguobacterium isolates from the Siberian permafrost. Description of Exiguobacterium sibiricum sp. nov. Extremophiles 10, 285–294 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

